Precipitation, Life, and Climate
Water appears to be a “simple” molecule consisting of two hydrogen atoms bonded to one oxygen atom, yet it has unusual properties compared to all others that make it vital for life to exist and thrive while also playing a critical role in moderating and regulating Earth’s energy budget. It is the molecule at the heart of Earth Systems since it is critically involved in all three of the big components: energy flows, matter cycles, and life webs. We often take it for granted since water is quite common on Earth and in the universe since nearly three-quarters of all molecules are hydrogen and just over 1% is oxygen, but a planet with all three phases (solid, liquid, and gas) readily available is not common.

The Mojave Desert, the driest place in North America, has minimal amounts of life compared to other ecosystems.
Water Properties Essential for Life
Humans are more than 50% water, and plants more than 90%. Water is essential for life, but why?
There are more than 70 known anomalous physical and chemical properties of water, many of which are essential for life to evolve, exist, and thrive. Here are some of the critical ones:
- Water has the highest specific heat of all liquids, and since living organisms contain so much water, their temperatures are quite stable. The rates of many biochemical reactions are temperature sensitive, so a steady temperature is essential for healthy life forms.
- Water’s latent heat of vaporization is the highest of all naturally occurring materials on Earth. Humans sweat to cool their bodies as the water evaporates from the skin. Dogs can’t sweat, but they pant to cool themselves as their saliva evaporates from their tongues. Similarly, human respiration also cools our internal temperature.
- Water is the ‘universal’ solvent (actually, nothing dissolves every material), so it carries dissolved nutrients and gases to cells within an organism and carries away waste materials.
- Water’s lack of compressibility plumps up cells to keep their shape, which maintains biochemical reactions within and around the cell.
- Water’s separation of positive and negative charges across the molecule (called polarity) helps DNA to produce its double helix shape and amino acids to fold so proteins become the proper shape. Without the shape of these essential organic molecules, cells would not function properly.
- Water may break down to produce H+ and OH¯, so it acts as a buffer within cells to maintain a steady pH for the biochemical reactions that are sensitive to acidic and basic conditions.
- Water is an essential component of photosynthesis in plants. Without plants, Earth would not have an oxygenated atmosphere – and no air-breathing animals.
- Water’s high cohesion, adhesion, and surface tension create capillary action that allows vascular plants, such as trees, to move water from the roots to foliage up to 300 feet away! The evolution of trees 400 million years ago transformed life on land.
- Ice, the solid form of water, is less dense than the liquid form. This allows animals and plants to survive in the water during the winter.
Numerous research institutes around the world focus on discovering more secrets about water. This section highlights a few of the properties that are essential for life but use the links if you would like to explore this fascinating topic further.
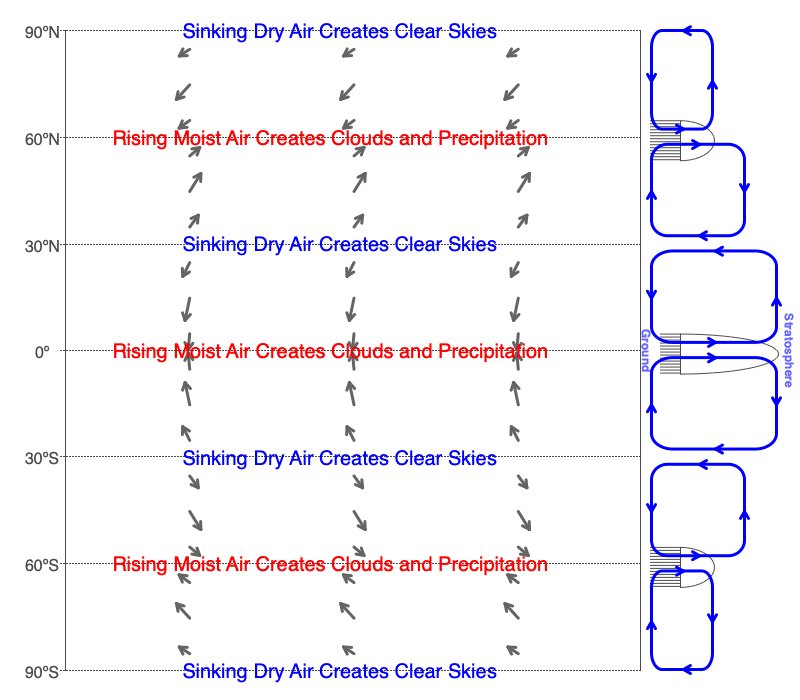
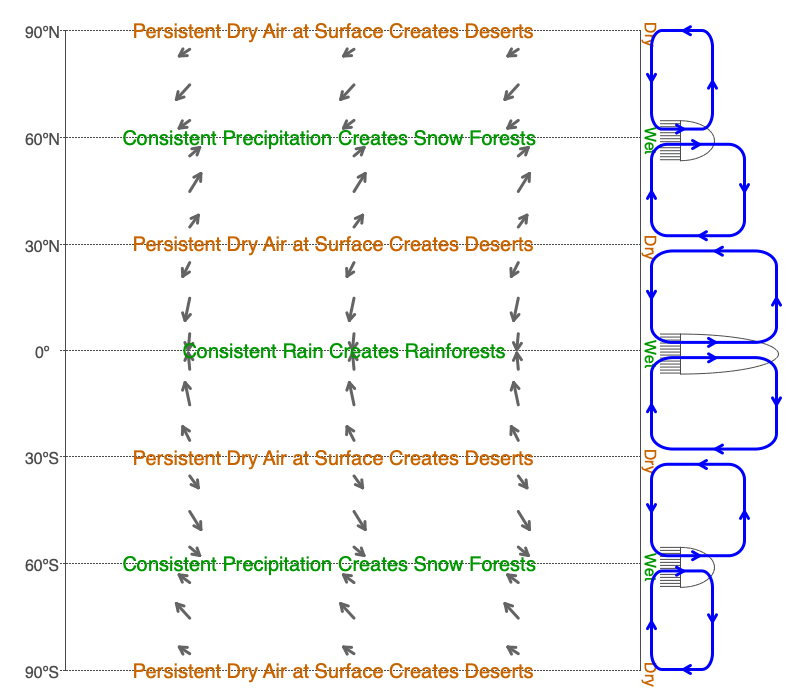
Consistent annual precipitation supports rain and snow forests. These areas have abundant life in an array of diverse forms. Whereas, the persistent sinking of dry air creates deserts that have minimal amounts of life. The global circulation cells create both of these climate regimes. Rising parts of the cells create consistent rains. The dry air leaving the tops of the rain columns sinks and creates deserts (see image above). Use the Global Winds section of the Earth, Wind and Forces software to explore these global bands of wet and dry conditions.
Notice the positive feedback mechanism between evaporation and precipitation within the circulation cells when they occur over the oceans. The copious precipitation creates a large supply of warm, dry air to sink at bands 30 degrees from the equator. This warm, dry air will evaporate more ocean water which feeds more water vapor to the bands of upwelling air at the equator and 60 degrees from the equator, making greater precipitation.
Deserts over Oceans?
A desert is an area with very little annual precipitation, and they aren’t confined to land areas. Even though there is plenty of water over the oceans, there are large regions with very little precipitation. Since only water evaporates from the oceans, salt minerals remain behind, making the surface waters denser. Precipitation is fresh water, so if it falls on the oceans, it mixes with the surface waters, decreasing the salinity.
Since the oceans are the primary source for precipitation over land, this means water is leaving the oceans but the salt stays. The precipitation over land helps break down rock and transport the chemicals to the oceans, and these chemicals become the salt in the oceans. This creates a positive feedback mechanism where the oceans become more saline over time. There is a threshold of ocean salinity for supporting life, but we know they haven’t exceeded this since complex life began 600 million years ago. It turns out that these ocean deserts help remove salt from the oceans – but only when these deserts are bodies of water with outflow to the oceans, such as the Mediterranean Sea and the Dead Sea today. The high salinity, high evaporation, and minimal precipitation create conditions where the salts chemical precipitate from the water and become rocks called evaporites. These evaporites remove salt from the oceans for long periods of time (residence time) allowing the oceans to stay within salinity values that support life.
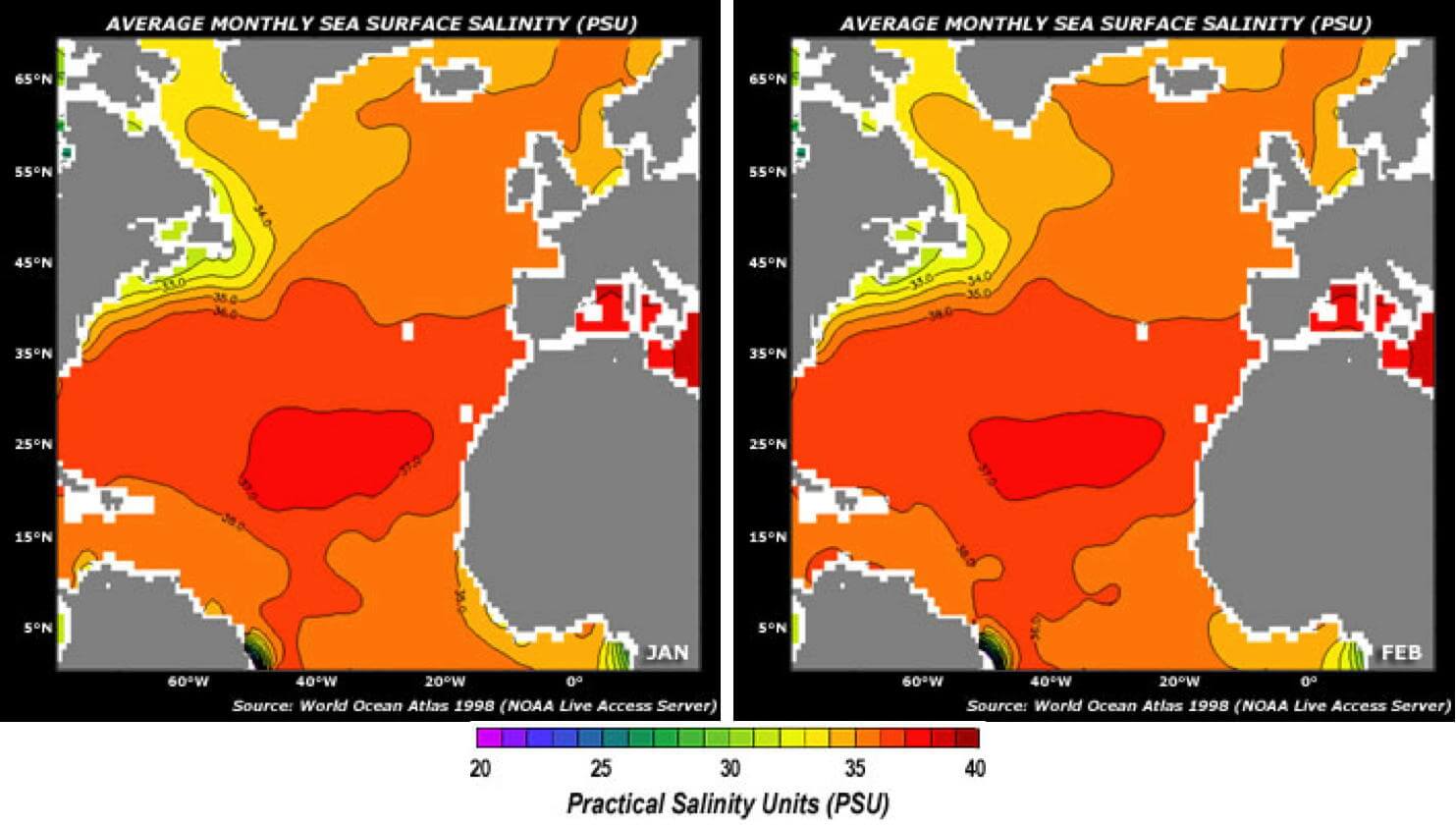
Salty Ocean Deserts
The oceans are not uniformly salty, as illustrated in the image above from NASA’s Aquarius Mission. Go to their site to compare contoured monthly data fields of a number of variables to determine the factors that create saltier and fresher waters in the Atlantic Ocean.
If not familiar with contour maps, see the Contouring activities.

0 Comments