Conduction
Temperature and Molecular Collisions
Temperature is a measure of the average kinetic energy of the atoms within a material, be it a solid, liquid, gas, or plasma. Because the individual particles are moving, they collide and transfer kinetic energy (0.5*mass*velocity*velocity) from those with more to those with less. If one part of the material has a higher temperature, conduction occurs as the more intense and frequent molecular collisions transfer energy away from the hotter area to the colder regions. Because the particles in solids are closer together than those in other phases, and they can’t flow away from each other, conduction is an essential mechanism for heat transfer within solids.
Conduction is also essential at phase boundaries: solid/liquid, solid/gas, and liquid/gas. Heat conducts across the boundary from hot to cold, and the fluid phases (materials that change shape (liquids and gases) may flow away from the boundaries involving advection and convection.
A temperature gradient is a difference in temperature over distance, so it is a vector. A vector is a quantity that has a magnitude and a direction. Think of baseball: the pitcher throws a ball toward the catcher, and the ball’s velocity is a vector. The magnitude is how fast the ball is traveling, and the direction is where the ball is heading. Both are important to recognize by the batter, pitcher, and catcher! Similarly, the temperature gradient determines how much conduction occurs and the direction of heat transfer.
Temperature gradients always point from hot toward cold, and heat conduction moves in the direction of the temperature gradient. If you completed the data contouring activities, the temperature gradient is always perpendicular to the isotherms, which are contours of constant temperature (“iso” means “same,” and “therm” means “temperature”).
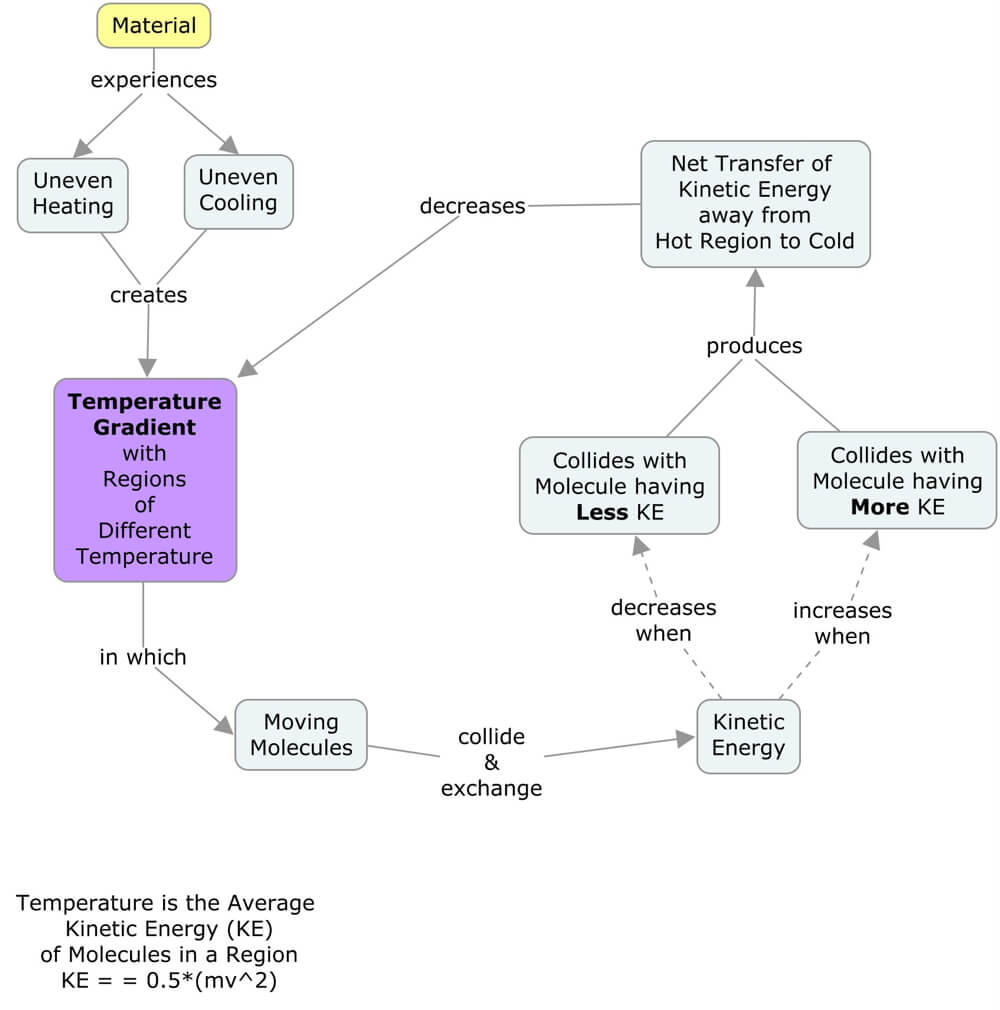
Concept map illustrating the macro-scale processes of conduction. Within solids, on a molecular scale, there are a number of additional factors to consider. In Earth systems, one of the connections we focus on is between energy flows and matter cycles, particularly matter cycles that involve the movement of fluids, which is why this simplified version of conduction is useful for our needs.
Acknowledgments
Mattea Horne and Mort Sternheim provided invaluable suggestions and insights in the many iterations of this concept map.
Run Virtual Conduction Experiments
Explore a model of heat conduction in a solid. Heat is the amount of energy flowing from one body to another spontaneously due to their temperature difference. Temperature is a measure of the average kinetic energy of the particles in a substance.
Any material with a temperature above absolute zero (0 K, -273.15°C, or -459.67°F) has moving molecules within it. The higher the temperature of the material, the faster the molecules move on average. In a solid, the particles vibrate about a fixed location within the solid. When a faster-moving particle collides with a neighboring slower molecule, a portion of the kinetic energy transfers to the slower one, so it moves faster. At the molecular level, this is the transfer of heat. When a portion of the solid is heated, the molecules within the area move more quickly. Particles in the colder region of the solid are moving slower, but molecular collisions will transfer heat from the warmer to the colder area.
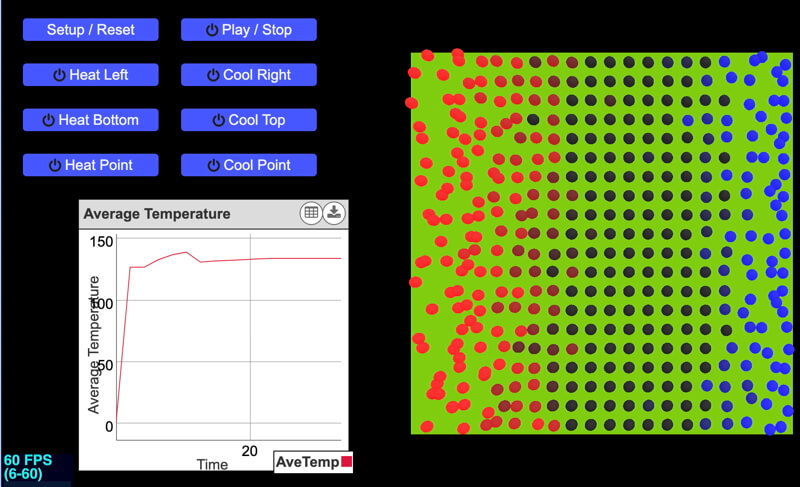
A screenshot of the Conduction Experiment interface. Reddish molecules are faster-moving molecules, bluish are slow, and dark molecules are moving at moderate speeds.
How to Run the Conduction Simulation
Step 1) Click “Setup / Reset.”
Step 2) Click “Play / Stop.”
Step 3) Create a temperature gradient in a variety of orientations and magnitudes by either heating and/or cooling parts of the solid square.
a) Click “Heat Left” to heat the molecules along the left edge of the solid.
b) Click “Heat Bottom” to heat the molecules along the bottom.
c) Click “Heat Point” to heat a small number of molecules along the center of the left side.
d) To remove heat from molecules on the edges of the solid, click the “Cool” buttons.
Create a number of experiments to see how heat conducts through a solid depending on the direction and magnitude of the temperature gradient (change in temperature over distance).
To reset the temperatures of the molecules for another experiment, click “Setup / Reset.”
This is an open-source app using MIT’s StarLogo Nova programming language. Bookmark this page, not the URL of the app since updates will be at different sites.
Acknowledgments
I would like to thank Lincoln Berkley, Mattea Horne, and Mort Sternheim for sharing their valuable suggestions to improve the model.
Big Ideas
- Temperature is a measure of the average kinetic energy of the atoms within a material.
- Conduction is the transfer of thermal energy away from hotter regions to colder areas through molecular collisions.
- Conduction transfers thermal energy in the direction of a temperature gradient, which is a difference in temperature over distance and points from hot to cold.

0 Comments