Ideal Gas Law and Clouds
Latent Heat and the Ideal Gas Law
A rising parcel of air without condensation occurring cools at 9.8ºC/km, and sinking air heats up at the same rate. This temperature change with height is the dry adiabatic lapse rate. When a cloud forms, the air parcel warms from the release of latent heat of condensation, changing the adiabatic lapse rate. Within a cloud, an ascending saturated parcel’s temperature decreases at roughly 5ºC/km. The moist adiabatic lapse rate is nearly half of the dry adiabatic lapse rate. To learn how to calculate the moist adiabatic lapse rate, see this article.
When air sinks inside a cloud, the cooling caused by evaporation decreases the rate of warming, so a sinking saturated warms at the moist adiabatic lapse rate. Once all of the liquid water has evaporated, the sinking air returns to warming at the dry adiabatic lapse rate. If the rise and fall of an air parcel are adiabatic (no exchange of heat and mass with the surrounding air), the temperature and dew point of the parcel will be the same when it returns to the ground, even when condensation occurs.
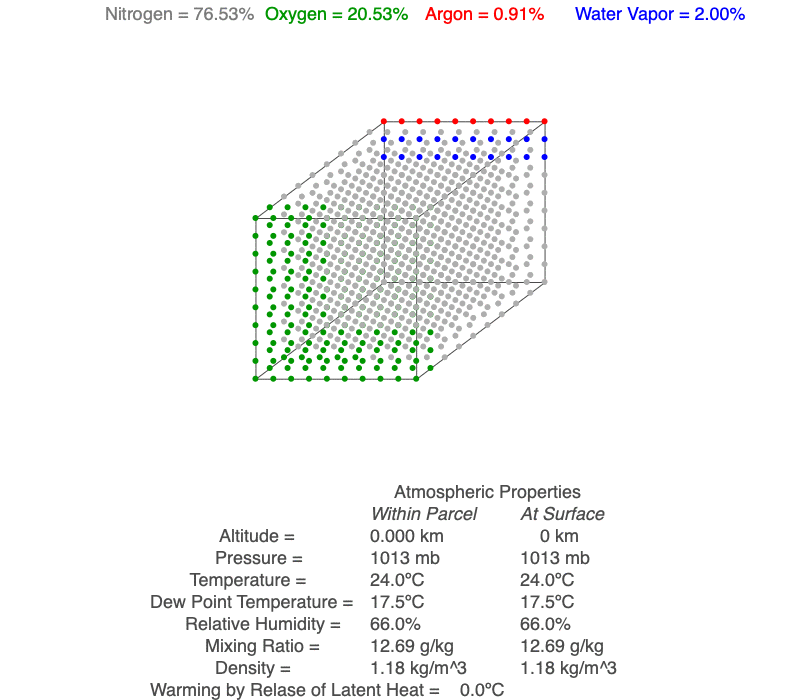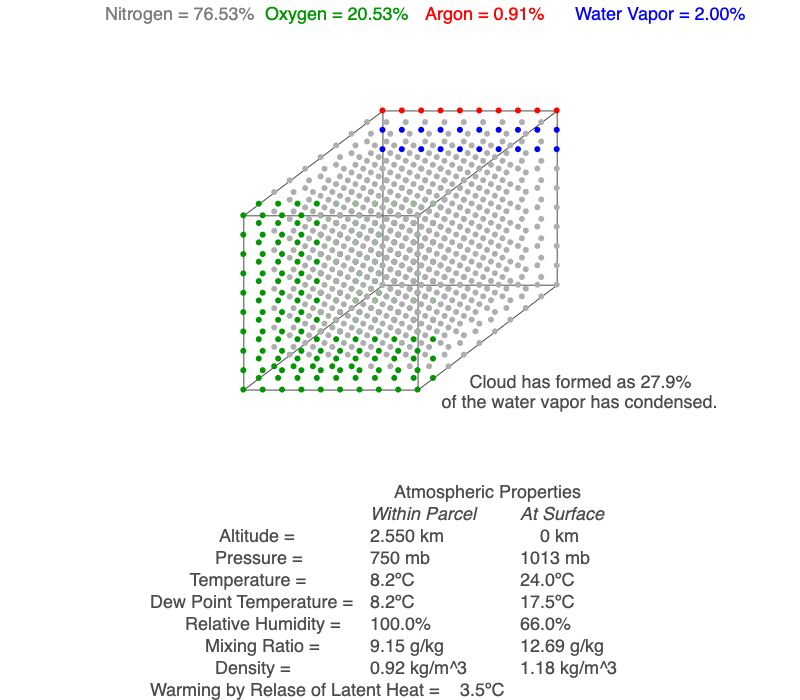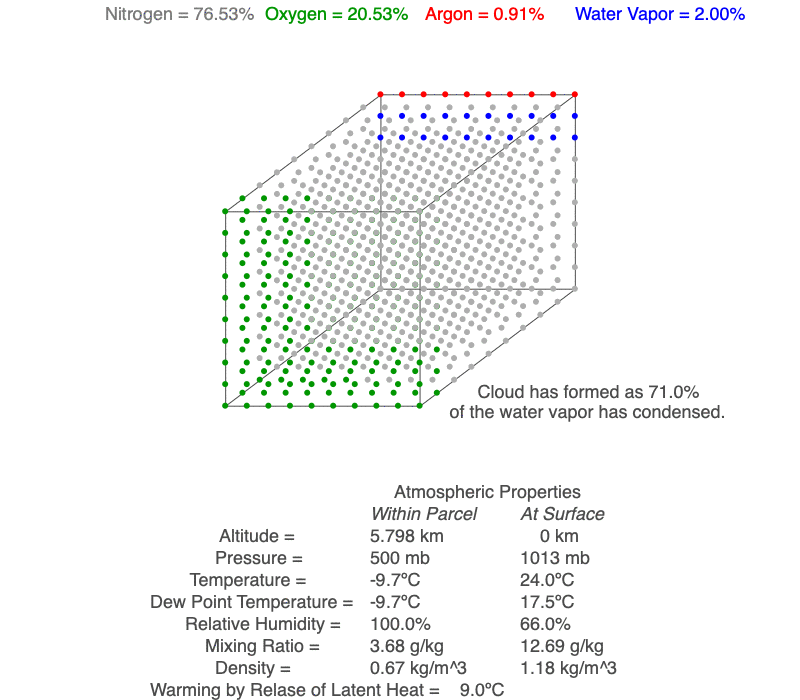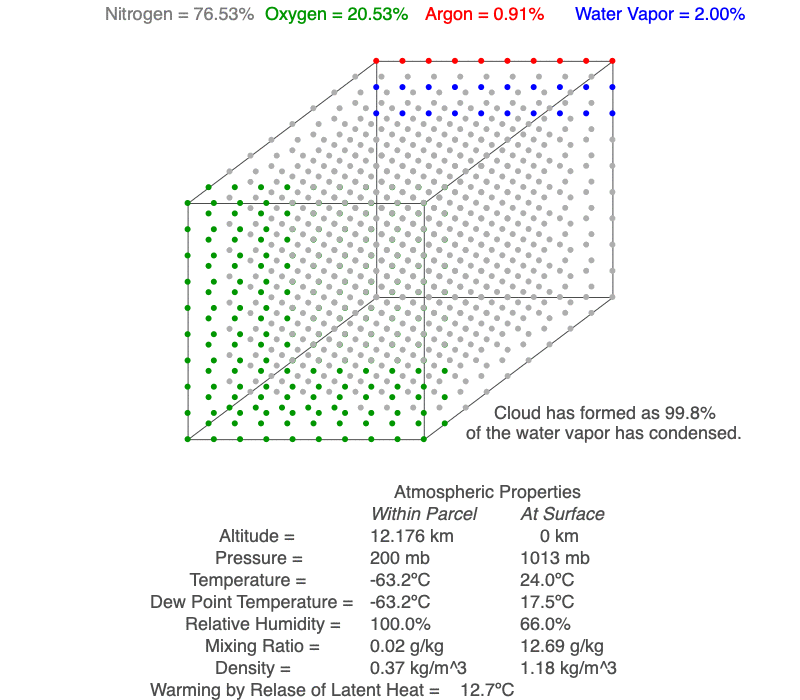
This air parcel has 2% of its molecules as water vapor (blue). As the rising air cools, the water vapor will eventually condense, a cloud forms and latent heat of condensation warms the air parcel, so it cools less than for dry conditions. As the air continues to rise, more water vapor condenses.
Quanitifying Amount of Water Vapor
Water vapor is one of the gases in air parcels, and it ranges from nearly zero to up to 3% of the molecules. Due to the many processes water is involved in, meteorologists use a number of variables to describe how much water vapor is present in the air. We will use three of them for our exploration of cloud formation.
Dew point temperature is the temperature condensation begins within the air parcel. The greater the concentration of water vapor, the higher the dew point temperature, but it is always less than or equal to the temperature of the air parcel. Because increasing air pressure increases the temperature at which condensations occurs, dew point temperature decreases nearly 2ºC/km for rising air.
Mixing ratio is the mass of the water vapor divided by the mass of the dry air. This value remains constant for rising and sinking unsaturated air.
Relative humidity is a combination of both temperature and dew point temperature, so it is useful for indicating how close the air parcel is to saturation, which occurs when RH = 100%.
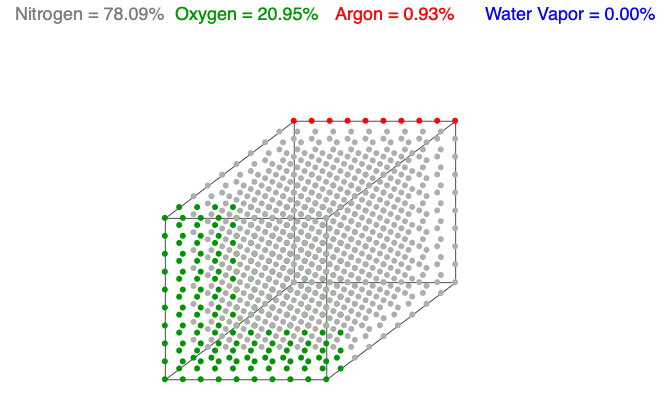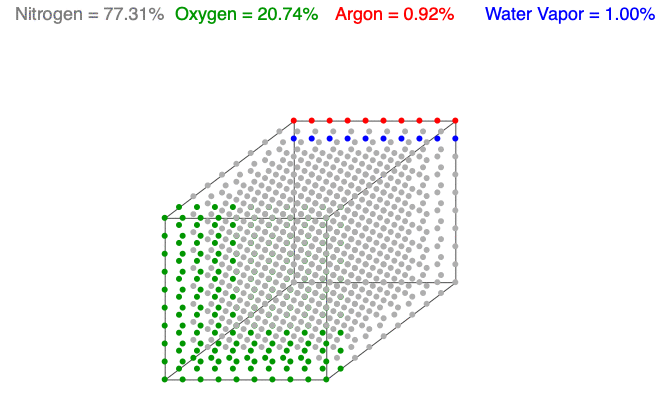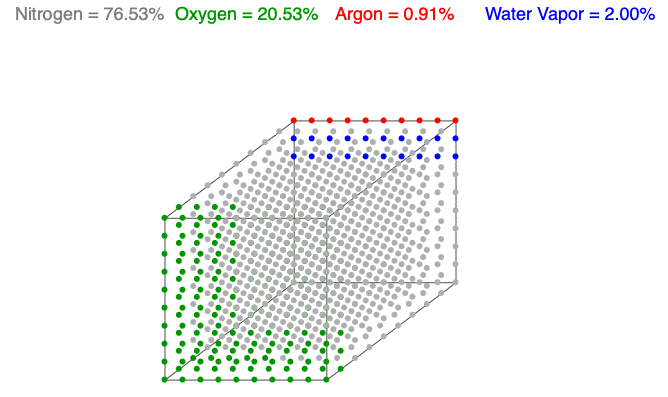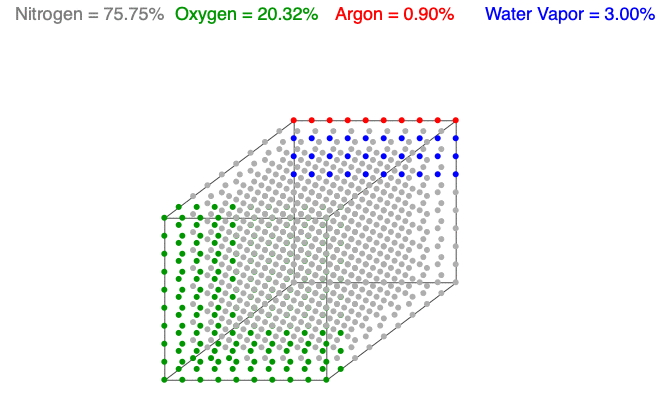
Above is an animation showing water vapor increasing in a unit volume of air. As water vapor (blue) increases, the amount of diatomic nitrogen (gray) and oxygen (green) decrease per unit volume, which lowers the mass and decreases the air’s density.
Water Vapor and Air Density
Water vapor (H2O) is the most variable molecule entering and exiting the atmosphere. It is one of the lightest molecules in the air, especially compared to the dominant gases, diatomic nitrogen (N2) and oxygen (O2). When water vapor increases, the amount of oxygen and nitrogen decrease per unit volume (the term n in the Ideal Gas Law remains constant), which lowers the mass and decreases the air’s density.
Two components determine air density: temperature and chemical composition, but the temperature has a dominant effect compared to the amount of water vapor (see the graph that follows).
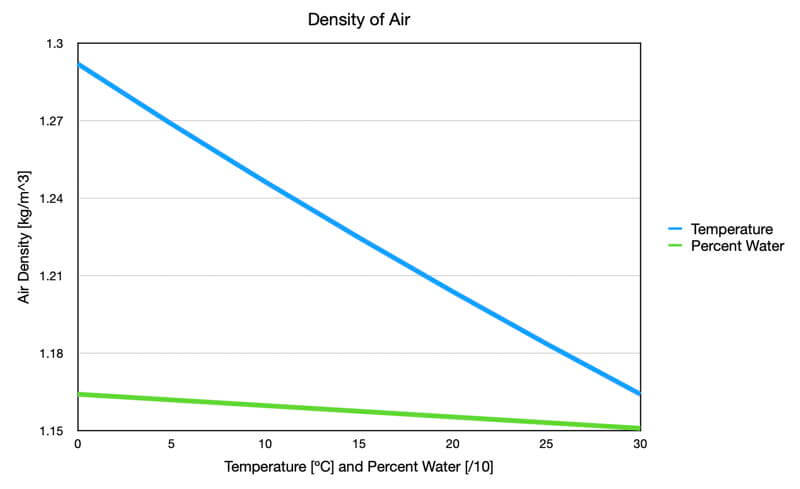
Graph showing the effect of temperature (blue) and percent water vapor (green) on air density. The temperature data used dry air, and the water vapor data used an air temperature of 30C. Both were at standard surface air pressure. Notice that the temperature has a dominant impact on air density.
Cloud Formation
As an unsaturated air parcel begins to rise, pressure decreases, the parcel expands, the temperature falls at 9.8ºC/km, dew point temperature decreases at 2ºC/km, and relative humidity increases. A cloud forms when the parcel’s temperature equals the dew point – this is the base of the cloud. If the parcel continues to rise, both temperature and dew point decrease at the moist adiabatic lapse rate (roughly 5ºC/km), and more of the parcel’s water vapor condenses as the cloud grows upward.

A cloud forms when the air parcel’s temperature (blue line) and dew point temperature (red line) become equal, which is also when relative humidity reaches 100%. After a cloud forms, both temperature and dew point change at the same rate (magenta line).
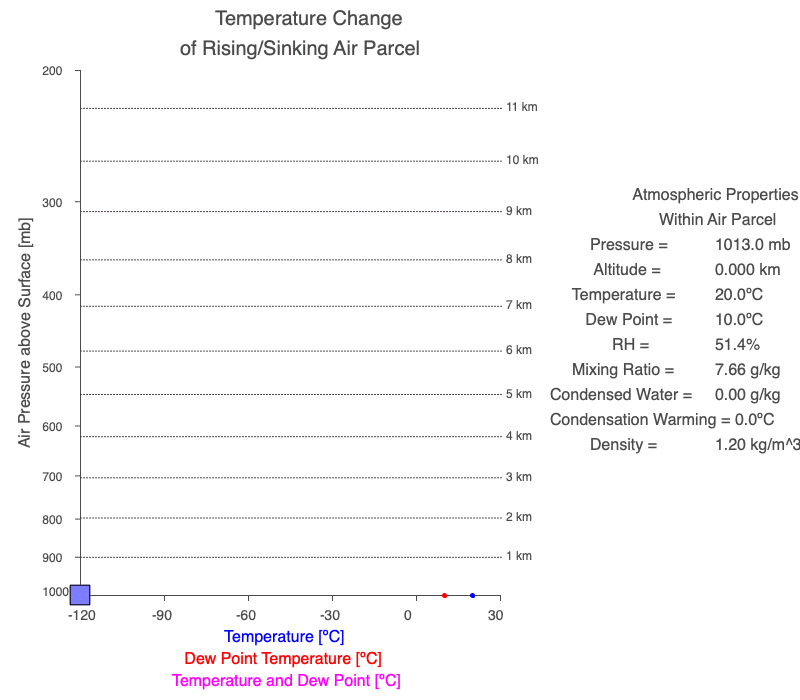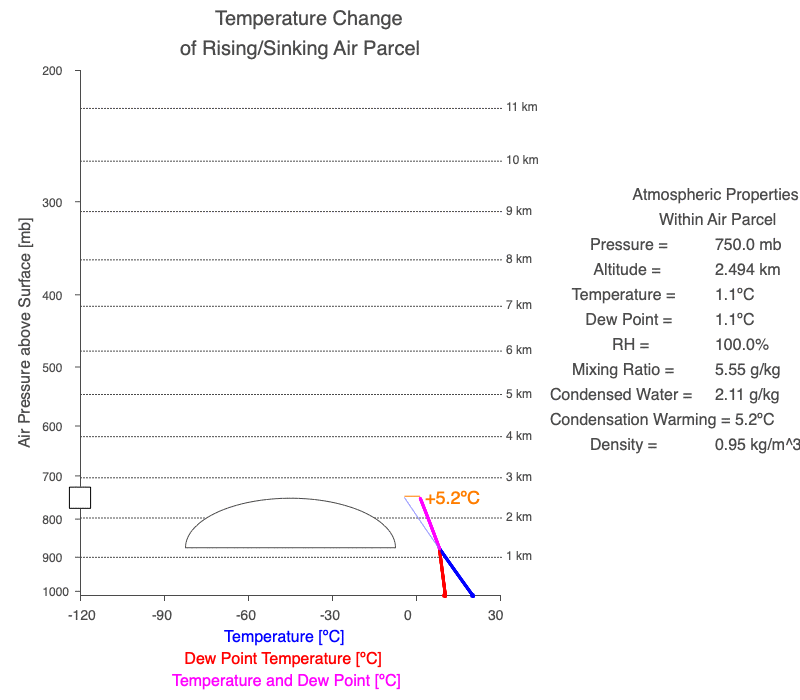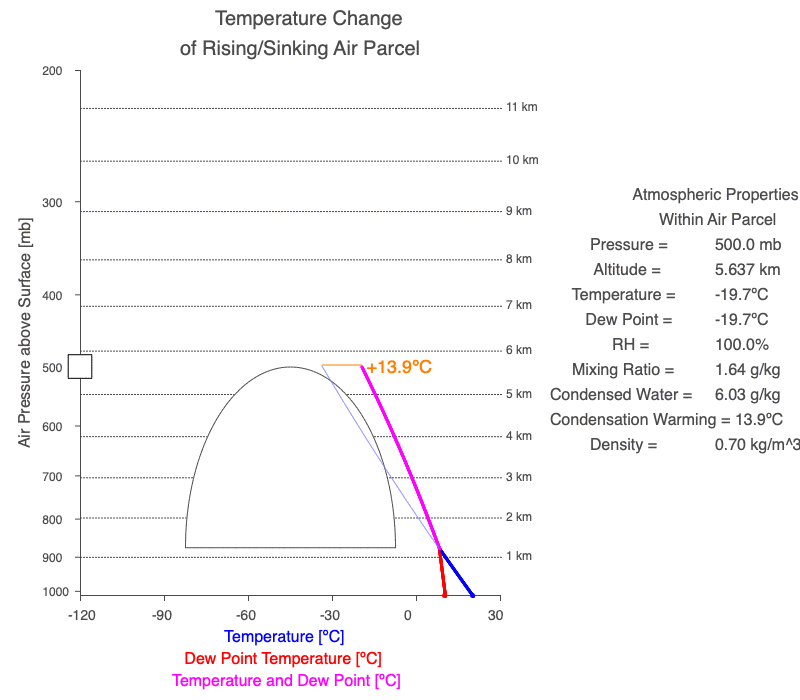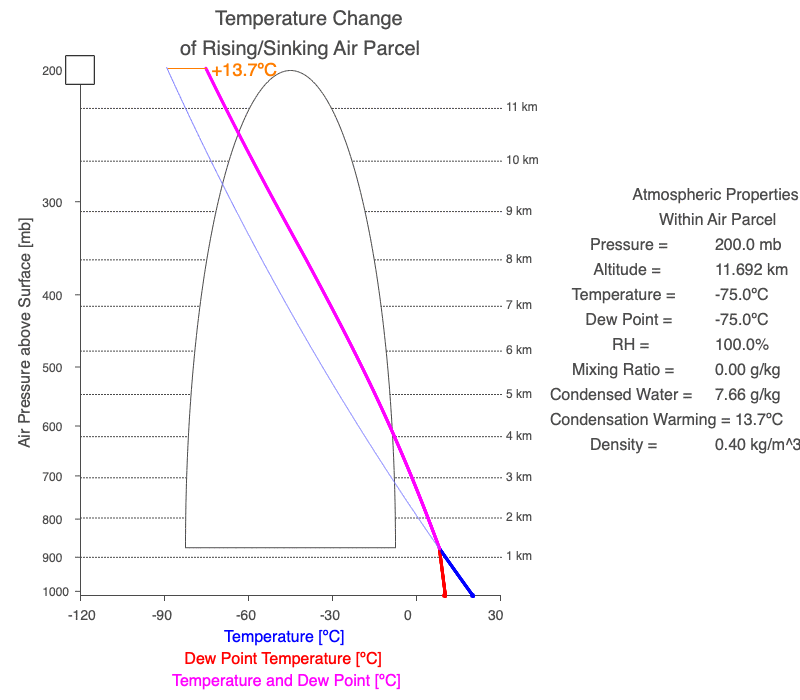
Follow a rising parcel as it moves vertically (located on the y-axis). It starts blue since there isn’t a cloud, and turns white when the cloud forms. When different, the parcel’s temperature is blue, and the dew point is red. When two values change at the same rate, the line is magenta. The thin blue line shows the parcel’s temperature if condensational heating didn’t occur. The orange value is the difference between the dry and moist adiabatic lapse rates at the parcel’s altitude, and it represents the effect of latent heat of condensation on the air parcel.
Explore the Ideal Gas Law
Visit the Ideal Gas Law tab in the Atmosphere, Clouds, and Precipitation software to explore the connections between the Ideal Gas Law and the formation of clouds. Go to the Software page to download a version for Macs or PCs.

0 Comments