Latent Heat
Latent heat is the energy needed to be released or gained by molecules within a material to change phase. A substance’s phase is determined by the relationship between the energy of attraction between the molecules (known as the energy associated with the intermolecular forces or IMF) and the kinetic energy of the moving molecules (KE).
The strength of an intermolecular bond is proportional to the electrical attraction between the molecules (recall, opposite electrical charges attract) and inversely proportional to the square of the distance between the molecules’ charges. So the IMF increases rapidly as the molecules get closer together. Temperature is proportional to the average kinetic energy of the moving molecules within an object. As a material’s temperature increases, its faster-moving molecules move further apart from each other, IMF decreases, and the material’s density decreases.
| Phase | Energy Associated with IMF Compared to Kinetic Energy of Moving Molecules | Freedom of Movement by the Molecules | |
| Solid | Energy(IMF) > Average KE | Most Restricted | |
| Liquid | Energy(IMF) ≈ Average KE | Some Restrictions | |
| Gas | Energy(IMF) < Average KE | Unrestricted |
Table showing the comparison between the energies associated with IMF and the motion of the molecules (KE) and the amount of freedom of movement by the molecules in the phases of a material. The molecules in a solid are not free to move past each other; they vibrate and spin within a restricted space. Molecules in a liquid have less restricted movement, and they slide past one another. In a gas, molecules are free to move in straight-line motion until they collide with other molecules.
For a material to change phase, energy is given up or taken in to create the conditions for the new phase to exist. Transitioning to a phase with more freedom of molecular motion requires that kinetic energy must increase to move the molecules further apart and decrease the energy associated with the IMF. The needed energy comes from the surroundings, thereby cooling it. These are endothermic phase changes.
To transition to a phase with less freedom of molecular motion requires that kinetic energy must decrease to allow the molecules to get close enough for the intermolecular force to change the type of molecular motion. The molecules give off this excess energy to the surroundings, warming it. These are exothermic phase changes.
As a material undergoes a phase change, heat is being gained or lost, but its temperature is not changing. Only after the material has wholly changed phase can its temperature change as heat is exchanged with its surroundings. So a more refined definition of latent heat is that it is the amount of heat required to create a phase change without a change in temperature.
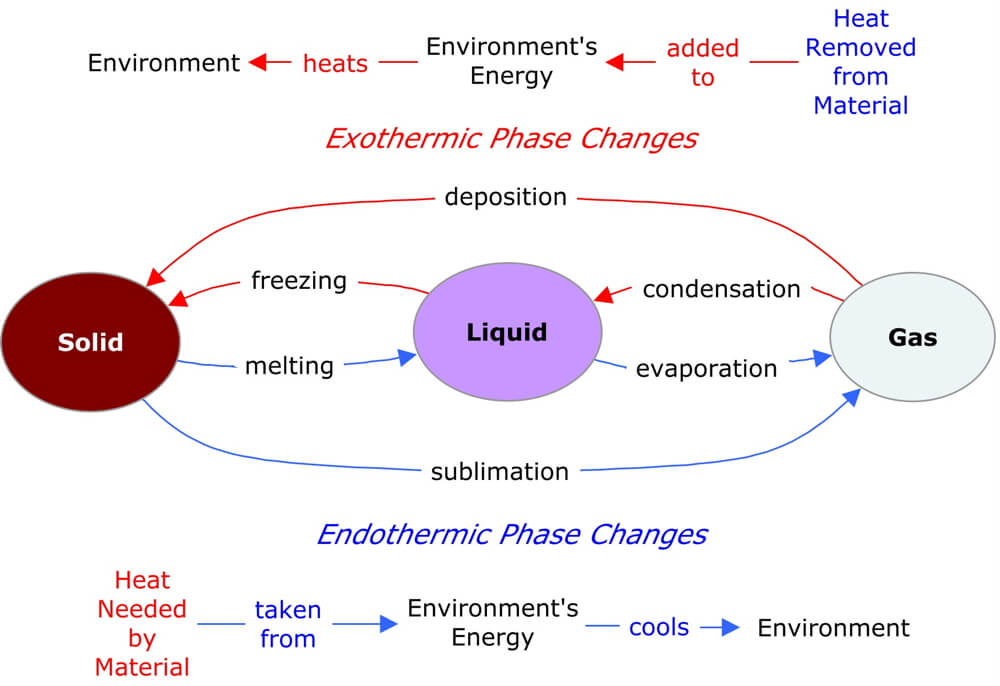
This diagram summarizes the six types of phase change and how heat is transferred between the material and the surrounding environment.
Acknowledgments
Max Hall and Mattea Horne provided invaluable suggestions and insights during “heated” exchanges to create this version of the concept map.
Hand Warmers: The Best “Hands-On” Activity!
Most of us use the term freezing in our everyday life to talk about icy conditions. Therefore, recognizing that the changing of a liquid into a solid, or the process of freezing, gives off heat is counterintuitive.
Use reusable hand warmers, such as HotSnapZ, for one of the most memorable hands-on experiences that freezing gives off heat! This is a hands-down favorite activity – just make sure everyone who is participating gets their own to hold until it is completely solidified (frozen).
Similarly, we tend to use the term melting when conditions are hot. But put an ice cube in your drink, and as the solid changes to a liquid, our beverage cools.
Importance of Phase Changes of Water
Water is quite common on the Earth’s surface and in its atmosphere. Water’s extraordinary chemical and physical properties help it play a critical role in heat transfer in the oceans, land, and atmosphere. In particular, its latent heat of condensation and evaporation is the greatest for materials found in nature.
Phase Changes that Cool the Surrounding Environment
-
- Sublimation -> Takes 620 cal for every gram of ice that sublimates. Without melting taking place, snow cover may decrease due to sublimation.
- Melting -> Takes 80 cal for every gram of ice that melts. Examples in nature include when glaciers melt to form streams.
- Evaporation -> Takes 540 cal every gram of water that evaporates. Since roughly three-quarters of the Earth’s surface is water, evaporation from the vast oceans is extensive.
Phase Changes that Warm the Surrounding Environment
-
- Deposition -> Releases 620 cal for every gram of ice forming from the vapor. This occurs in nature when frost and snowflakes form.
- Freezing -> Releases 80 cal for every gram of ice forming from water. Examples in nature are when the surface water of a pond freezes during cold winter months and when hail forms in severe weather events.
- Condensation -> Releases 540 cal for every gram of water forming from the vapor. This happens when dew forms on cool mornings and when clouds form in rising moist air.
For reference, a U.S. dime weighs 2.268 grams.
Latent heat released during phase changes of water plays a variety of essential roles in Earth systems. For one, it helps create more intense storms in our atmosphere, and it also increases how much heat is transported to the polar regions from the tropics via global atmospheric circulation cells.
Example of phases of water in nature.



Heating Curves
When thermal energy is being added to a solid, its temperature increases until it begins to melt. At this point, the solid remains at a constant temperature as more and more of the material melts into a liquid. Once melted completely, then the liquid’s temperature may begin to rise. When the liquid starts to boil, the liquid remains at a constant temperature until all of the liquid has transitioned to a gas, at which point the gas’s temperature begins to rise.
If you’ve played with an iced drink by putting in food coloring, you know that a drink with ice cubes floating near the top creates convection cells within the liquid below. The melting ice cubes are cooling the adjacent liquid, causing it to sink, and it is replaced by warmer liquid. The ice cube remains at a constant temperature (its melting point) until all of it is melted.
Acknowledgments
Will Tucker provided “cool” insights to include heating curves to this webpage.
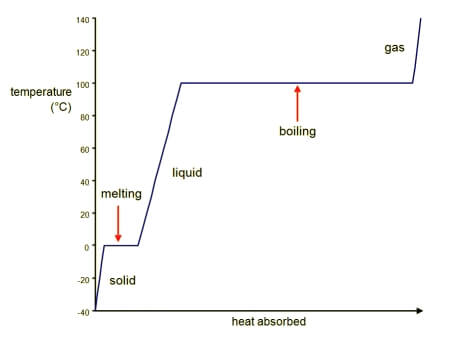
A graph of the heating curve of a substance starting as a solid and ending up as a gas. Image from Boundless Chemistry. Provided by Boundless Learning.
Offsetting the Rates of Heating and Cooling
A phase change affects the temperature of its surroundings, which affects the rate at which the material itself is heating or cooling. In the following concept maps, as a material warms and transitions to a phase where the molecular movement is less restricted, the heat taken from the surroundings cools it. The cooler surroundings ultimately slow the rate of heat entering the material. Similarly, cooling a substance so that it transitions to a phase with more restricted molecular movement releases heat and warms the surroundings, thereby slowing the rate of cooling of the material.
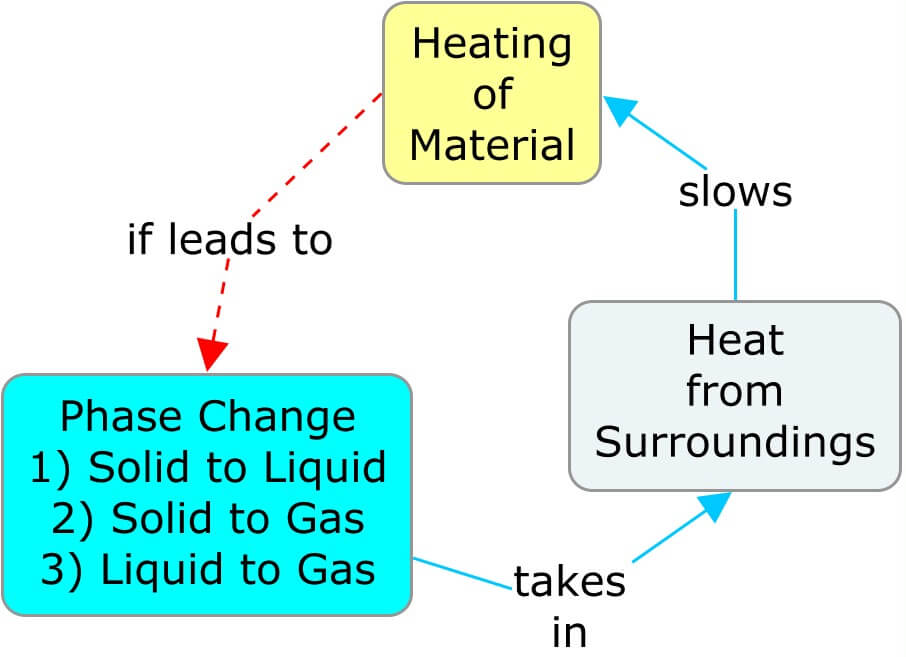
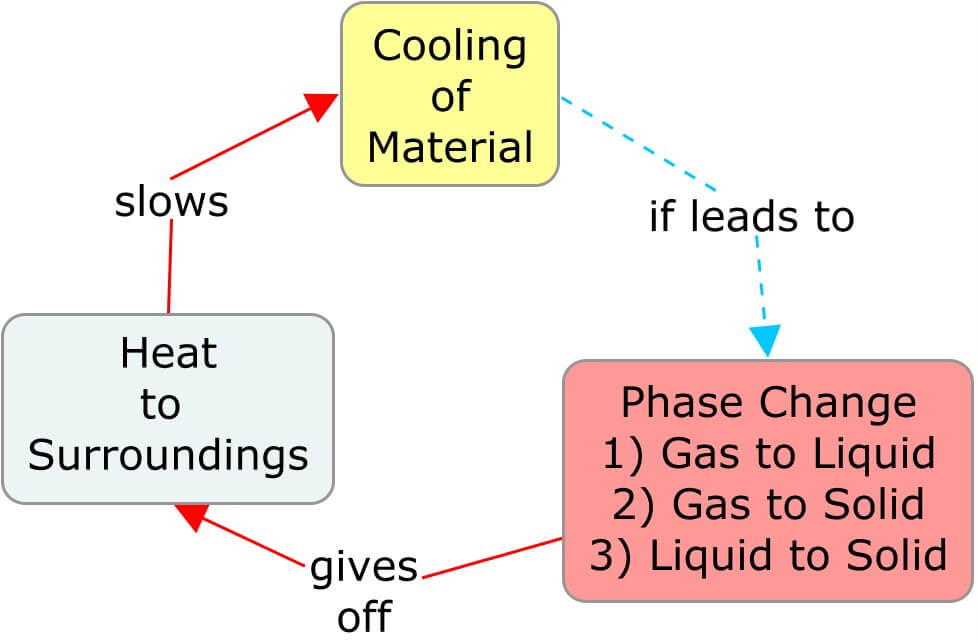
Big Ideas
- Latent heat is the amount of heat required to create a phase change without a change in temperature,
- The magnitude of latent heat depends proportionately on the bonding strength of the intermolecular forces (IMF) associated with the material’s phase that is changing.
- For a transition from one phase to another to occur, energy is given up or taken in to create the conditions for the new phase to exist.
- Transitioning to a phase with more freedom of molecular movement requires energy to increase the kinetic energy, which weakens the strength of the intermolecular forces as the molecules more further apart. The needed energy comes from the surroundings, thereby cooling it. These are endothermic phase changes.
- To transition to a phase with less freedom of molecular motion requires that kinetic energy must decrease to allow the strength of intermolecular forces to increase. The molecules give off this excess energy to the surroundings, warming it. These are exothermic phase changes.

0 Comments