CRACL
CRACL is an acronym of the heat transfer processes: Conduction, Radiation, Advection, Convection, and Latent Heat. The term highlights the fact that the heat transfer processes rarely occur one at a time. Instead, they are highly interconnected, maximizing the elimination of temperature gradients in nature.
Much of Earth Systems focuses on processes acting on or near the Earth’s surface, so usually, there are several phases of matter present. Heat transfer within and between these bodies of matter are critical to understanding Earth’s matter cycles and life webs.
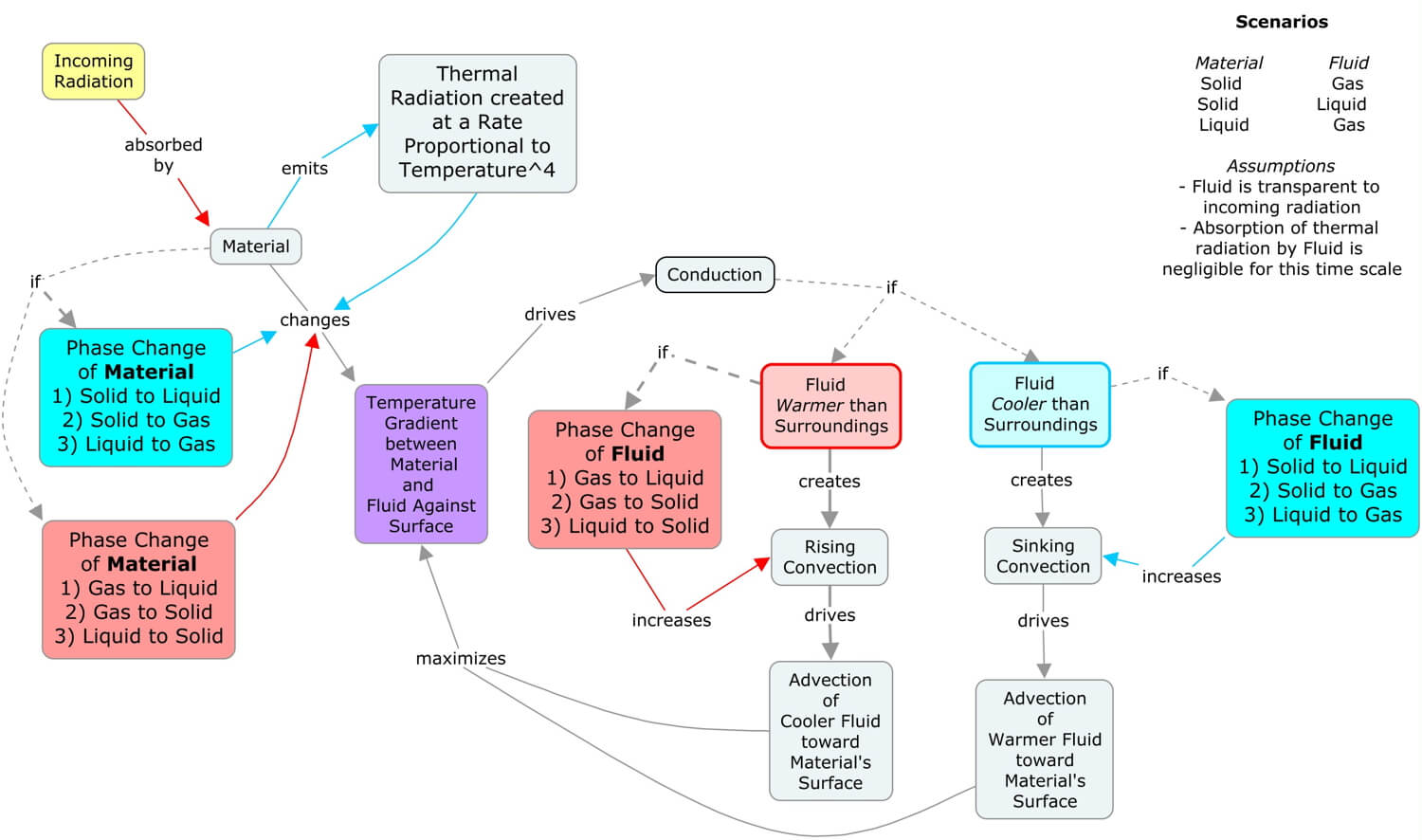
The concept map above illustrates how the five heat transfer processes (conduction, radiation, advection, convection, and latent heat, or referred to here as CRACL) interact in many of the scenarios of Earth systems. Since the Sun’s radiation drives many of Earth’s surface processes, start with the yellow “Incoming Radiation” concept, then follow the links to work through the interactions.
Collectively, the heat transfer processes are created and maintained by the Sun’s uneven heating of the Earth’s spherical surface. Nature is trying to balance this perpetual temperature imbalance, which as we will see in the following sections of Earth Systems, drives the matter cycles and sustains our complex life webs. The next section, the Sun-Earth Connection, explores the daily and seasonal patterns of the Sun’s illumination of Earth.
CRACL and Phases of Matter
The phase of matter determines which processes of CRACL are transferring the highest amount of heat.
Solid: Advection and convection cannot occur within a solid since the molecules cannot flow or change shape. Conduction is the primary mechanism for transferring heat within a solid. Most solids on Earth are opaque, meaning that radiation cannot transmit into the material. Instead, it is either absorbed or reflected by the object’s surface. Unless the solid is ice, deposition and sublimation don’t occur.
Liquid: Because liquids flow, advection and convection are dominant processes transferring heat within a liquid. Due to liquids’ lower densities, radiation penetrates most liquids to some degree. Important to aquatic life, light reaches surprising depths in our oceans and lakes. Water, the most common liquid on Earth, evaporates relatively quickly in many of the planet’s climate regimes, especially the tropics and midlatitudes.
Gas: Because they flow, advection and convection are usually the dominant processes transferring heat within gases. Due to their low densities, many gases are mostly transparent to the Sun’s radiation. The principal exception is ozone, which absorbs the Sun’s ultraviolet radiation. Greenhouse gases, which absorb mid- to thermal infrared emitted primarily from the Earth’s warmed land and oceans, are also critically important to Earth systems.
Water vapor, the gas phase of water, warms the atmosphere when clouds form during condensation and deposition.
Note: Of CRACL, only radiation passes through a vacuum. The other heat transfer processes require the matter to function.
CRACL and Phase Boundaries
Heat isn’t only transferred within an object; it is also transferred between objects. If the two objects have different phases, their combined properties influence how the processes of CRACL interact.
Solid-Liquid Boundary: The surface of the dense solid emits thermal radiation into the liquid, which may or may not absorb the radiation. If the two phases have different temperatures, significant conduction occurs at the phase boundary. The liquid may warm or cool, and depending on how the liquid changes density and the slope of the boundary, it may flow away, bringing in unmodified liquid to maintain the maximum rate of conduction possible at the boundary. This coupling of advection and convection sets up either a convection cell (two horizontal components of advection and two vertical columns of convection) or half a convection cell (only one section of advection and one of convection). When both phases are the same material, if the solid melts to liquid or the liquid freezes to a solid, then the effect of latent heat must be considered.
Solid-Gas Boundary: This boundary has similar heat transfer mechanisms as the solid-liquid boundary.
Liquid-Gas Boundary: Similar heat transfer processes to the other two boundaries, except for one big difference: convection cells often develop in both the liquid and the gas.
Whenever present, convection cells maintain the highest possible temperature gradient across the phase boundary, since unmodified fluid replaces fluid modified by conduction. This optimized temperature gradient maximizes the rate of conduction across the phase boundary.
Design Challenge
There are quite a few innovations to keep our drinks hot or cold longer. Explore which of the heat transfer processes were minimized in designing the container.
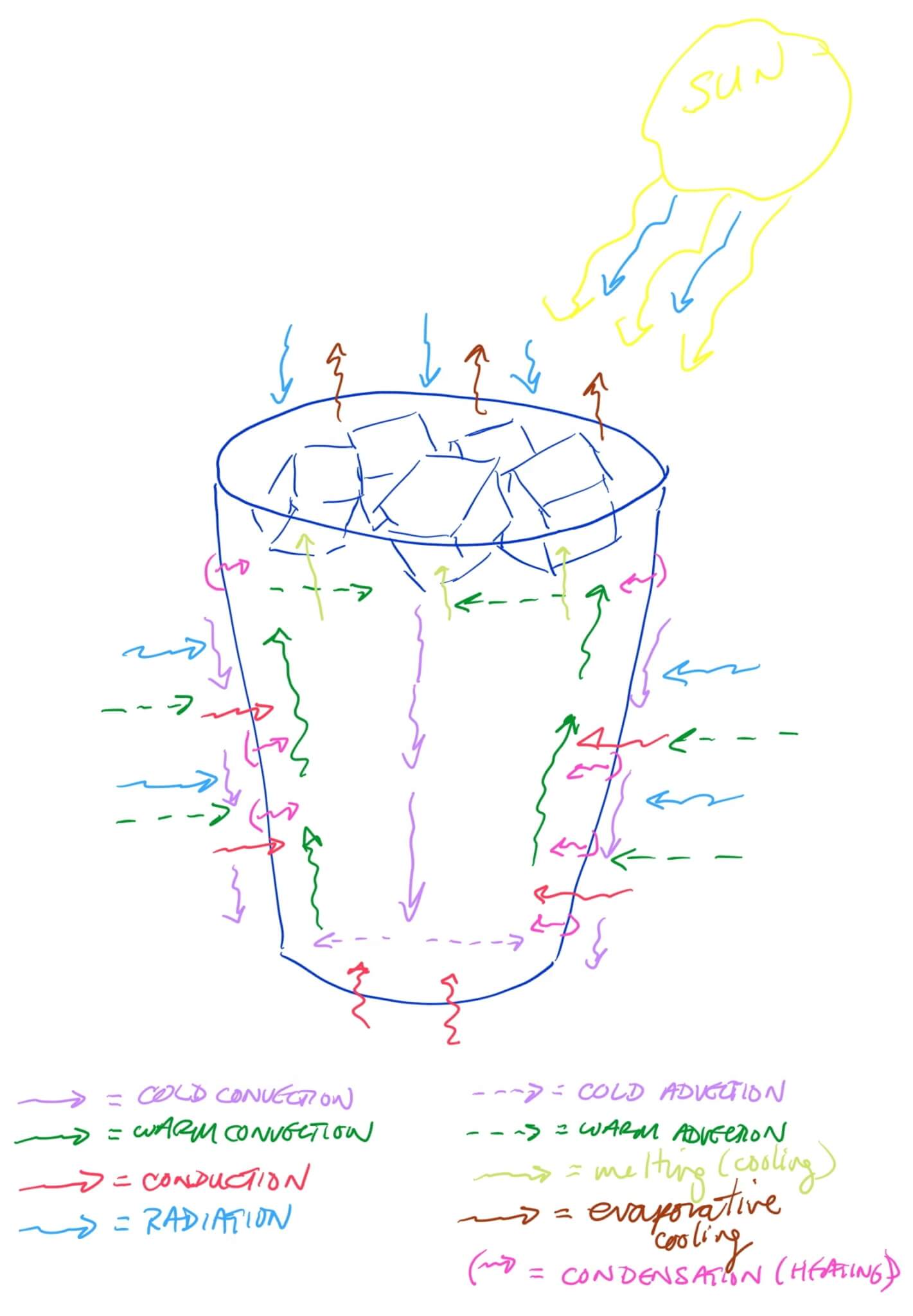
Above is a sketch of how an iced drink responds to sitting outside on a hot, sunny, summer day. Exchange of heat across the phase boundaries influences how the convection cells form within the liquid.
How many phase boundaries are there?
Seven, although six boundaries influence much of the heating of the iced drink.
- Ice cube (solid) – Liquid
- Glass – Liquid
- Inside the glass container
- Droplets on the glass on the outside of the container
- Glass = Table
- Glass = Atmosphere
- Ice cube (solid) = Atmosphere
- Liquid – Atmosphere
- Liquid at the surface within the container
- Droplets on the glass outside the container. Note that the evaporative cooling and resulting convection cells within the droplets, emitted thermal radiation, and conduction of heat to the atmosphere are not drawn due to the limited space available.)
- Ice cube (solid) = Glass (most likely minimal direct contact)
CRACL and Energy Budgets
Energy budgets are the rates of energy coming into and going out of objects ranging in size from an electron to that of galaxies. An object’s total energy increases when the rate of energy gained is greater than that being lost and decreases when the rate being lost is greater than that being gained. But there are times when the rates of incoming and outgoing energy are balanced, and the object’s total energy remains constant. To understand the energy budget of various components of Earth’s systems (or any scenario you are trying to make more energy efficient) work through the combined processes of CRACL.
CRACL and a Lava Lamp
Watch how conduction, radiation, advection, convection, and latent heat create the mesmerizing patterns in a lava lamp.
Practice Scenarios to Analyze with CRACL
Identify and label the effects of conduction, radiation, advection, convection, and latent heat acting on the temperature and behavior of the tea and its surroundings.
-
- A mug of hot tea sits outside on a bench in the shade on a cold winter night.
- A glass of iced tea sits on a bench in the sun on a hot summer afternoon.
Compare your results with these drawings:
Hot Tea and Cold Milk Experiment
As the class completed our exploration of CRACL, a student asked, “Which would be cooler? Hot tea and cold milk sitting separately on a table added together after 10 minutes, or hot tea with cold milk added immediately and left to cool for 10 minutes?” So we ran this experiment to find out. The volumes and starting temperature for both samples of hot tea and cold milk were identical, and the liquids were poured into identical paper cups.
The two drinks were about 15ºF (8ºC) apart after the 10-minute experiment – not a trivial difference! Which one do you think was hotter?
Work out the components of CRACL to support your answer. See how your answer compares with the results of the actual experiment.
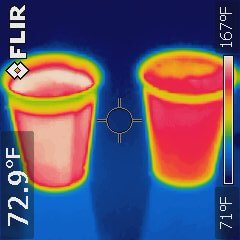
The thermal infrared image of the drinks after 10 minutes of cooling shows that the drink on the left is about 15ºF (8ºC) warmer. Which one was it in the experiment?
Big Ideas
- To understand what drives many Earth processes, consider the five heat transfer mechanisms acting on and between the objects that make up the system.
- Heat transfer processes rarely occur in isolation since there are usually two or more phases of material with different temperatures involved. Instead, they are highly interconnected, maximizing the elimination of temperature gradients in nature.
- The majority of heat transfer processes in Earth’s systems are created and maintained by the Sun’s uneven heating of the Earth’s spherical surface. Nature is trying to eliminate this perpetual temperature imbalance.

0 Comments