Variables Affecting Diurnal Heating
As we saw with the Black Fabric Experiment (see a discussion in Radiation Budgets), materials may have quite different diurnal heating patterns when exposed to the same intensity of sunlight. The first possible difference is reflectivity: some materials are more reflective than others. Objects that have a higher reflectivity gain less energy from sunlight compared to more absorbent materials.
Not all of the absorbed radiation goes directly into raising the temperature of the object. The temperature of an object represents the average kinetic energy of the molecules. However, molecules have other forms of energy, which are accounted for in the internal energy of the object. Materials have a property called specific heat, which is a measure of the amount of heat required to raise the temperature of a 1 kg mass by 1ºC. A material with a high specific heat needs more heat to raise the temperature compared to a substance with low specific heat.
We also need to consider the density of the material. For a given amount of absorbed radiation, a low-density material heats more quickly than a higher density substance.
Controlled Experiments
Using the Radiation Budgets and Make a Material options in the Blackbody Radiation software, controlled experiments explore the diurnal heating patterns for materials as one variable changes during the experiment:
-
- Reflectivity,
- Specific heat, or
- Density.
Diurnal Heating and Reflectivity
As reflectivity increased from 0% to 90%, there was less warming of the surface, so there was a consistent decrease in the emitted thermal radiation. Since the transmission of radiation through the surface material is negligible, the percent of sunlight absorbed is 100% – percent reflected. As the reflection increased, the amount of absorbed radiation decreased, the surface temperature decreases, and the emitted thermal radiation decreased even more so the maximum daily temperature occurred later in the afternoon.
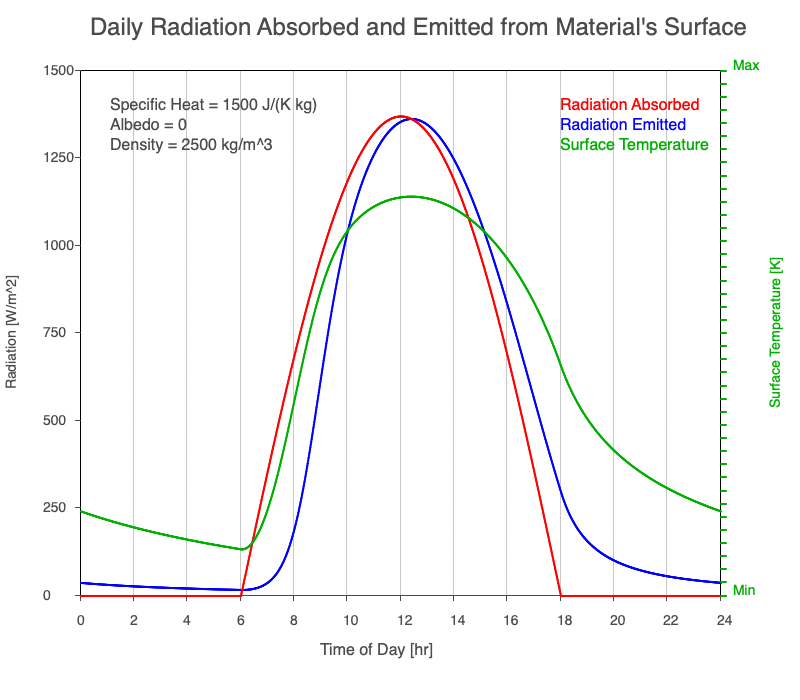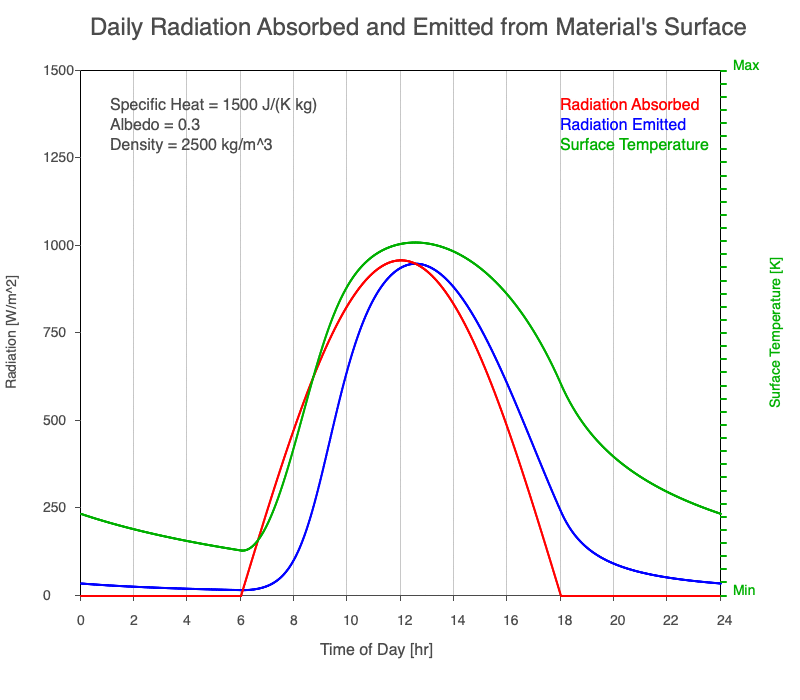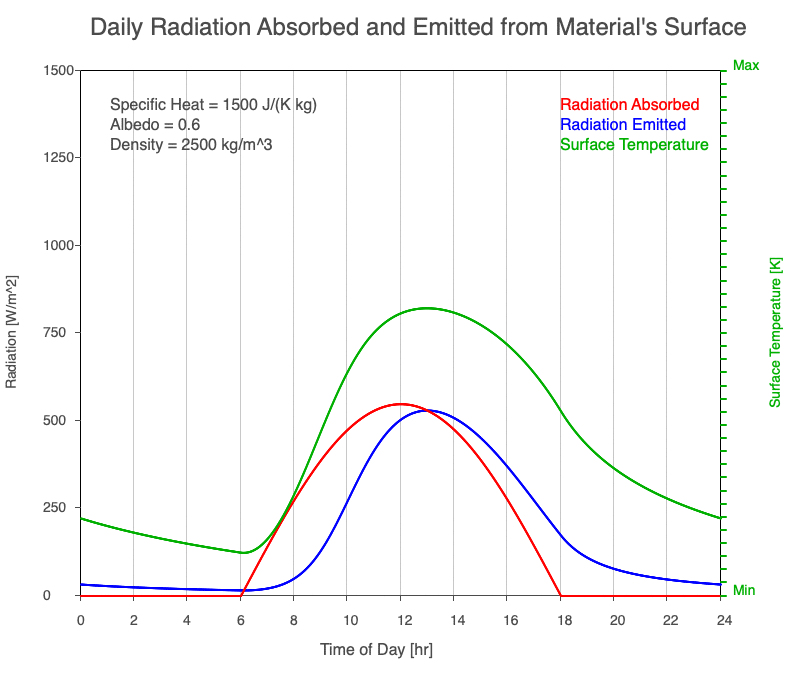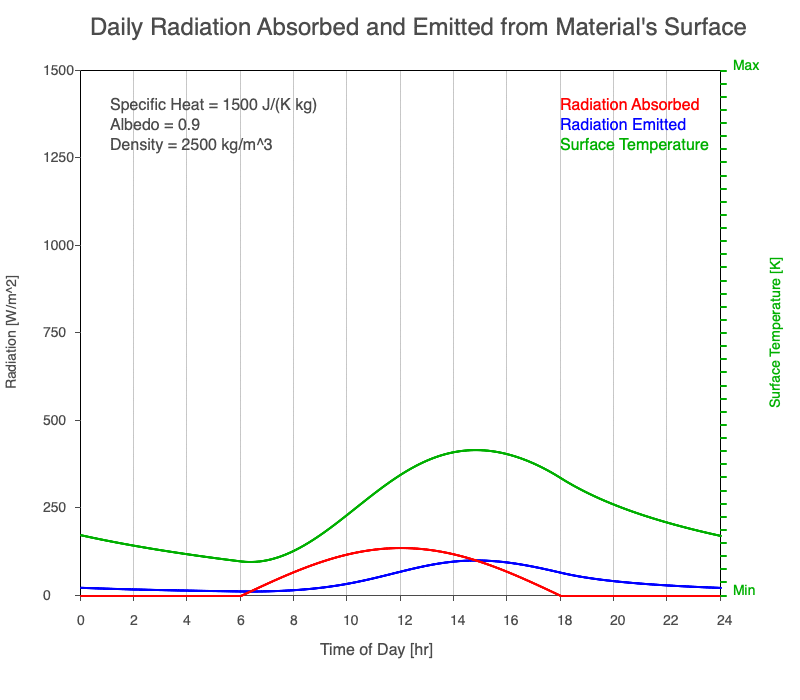
Animation of the effect of increasing reflectivity on the amount of absorbed radiation (red), emitted radiation (blue), and surface temperature (green) during a day with 12 hours of sunlight.
Diurnal Heating and Specific Heat
A material with a high specific heat warms less for a given amount of absorbed radiation compared to an object with lower specific heat. Since the amount of emitted thermal radiation depends on the material’s temperature, a material with high specific heat emits a smaller range in radiation during the day than a substance with lower specific heat if they absorb the same amount of solar radiation. Additionally, high specific heat materials have a more moderate and more delayed peak temperature during the afternoon.
Animation showing the diurnal heating as specific heat increases. All other variables are held constant. The red line is the absorbed radiation, the blue is the emitted radiation, and the green line is the surface temperature. The higher the specific heat, the more radiation needed to raise the temperature and the warmest temperatures occur later in the afternoon.
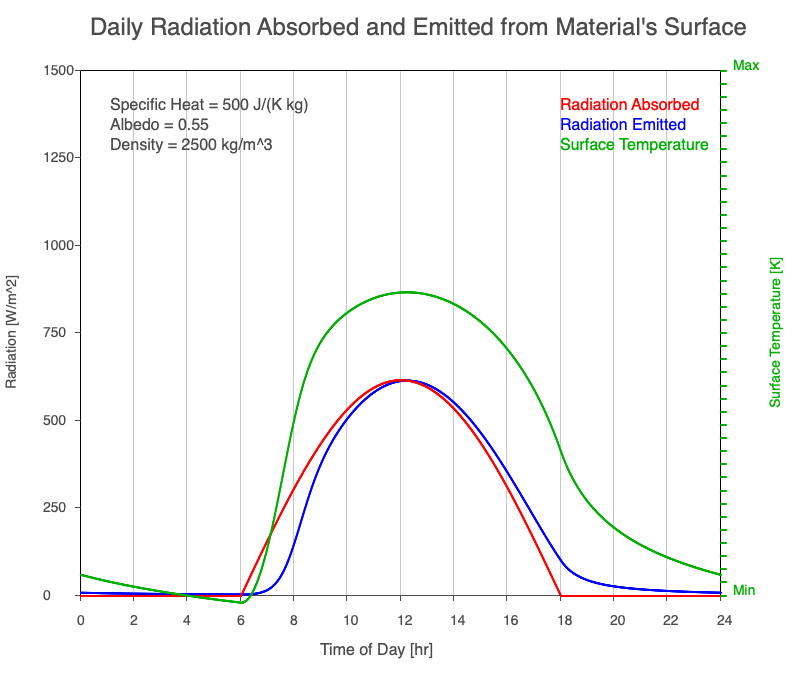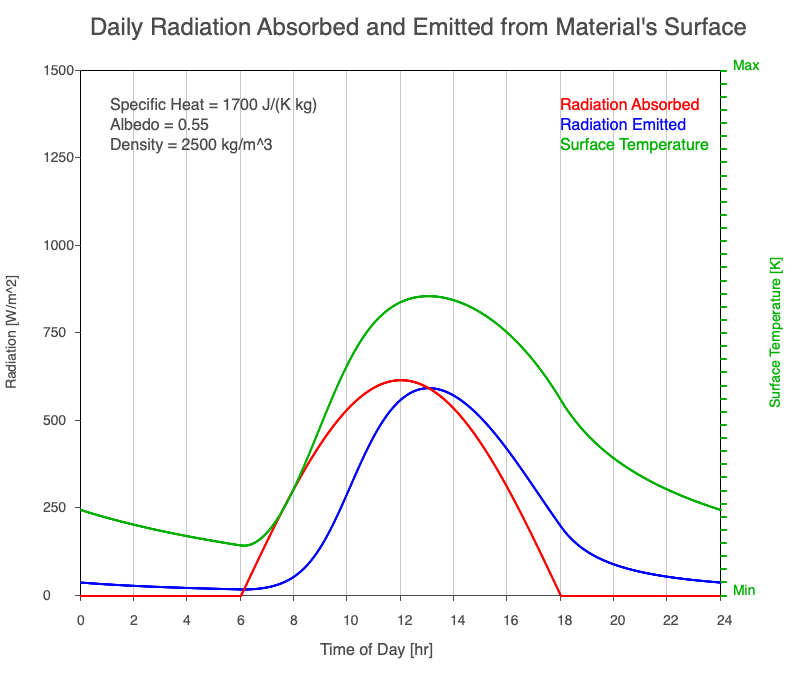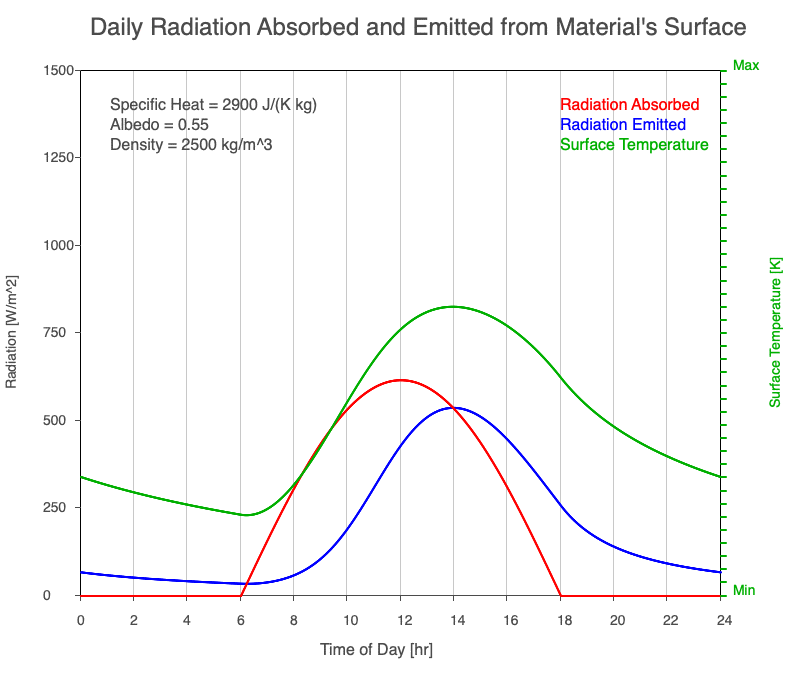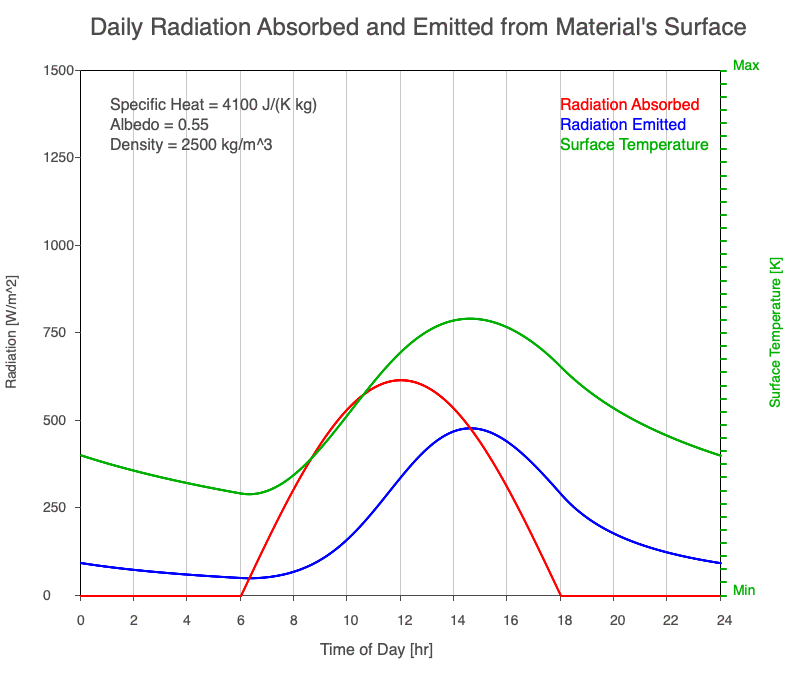
Diurnal Heating and Density
If a material’s chemical composition remains the same yet its density increases, there are more molecules within a given volume. As the number of molecules within a given volume increases, for a set amount of absorbed sunlight, the increase in average kinetic energy is less. As a result, the temperature doesn’t increase as quickly, which then decreases the amount of emitted thermal radiation.
Animation showing the diurnal heating as density increases. All other variables are held constant. The red line is the absorbed radiation, the blue is the emitted radiation, and the green line is the surface temperature. As density increases, for a given amount of absorbed radiation, there are more molecules to disperse the energy, so the temperature and emitted thermal radiation decrease.
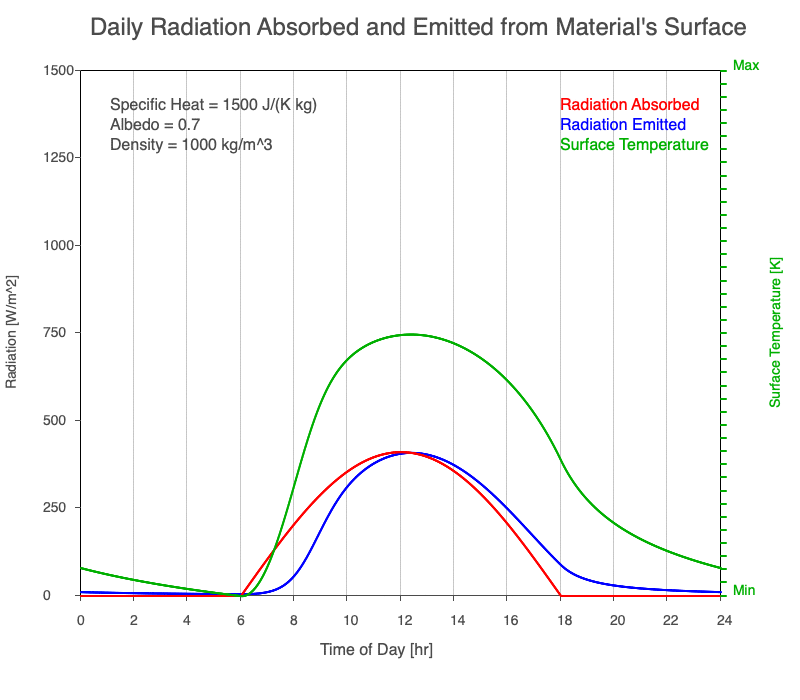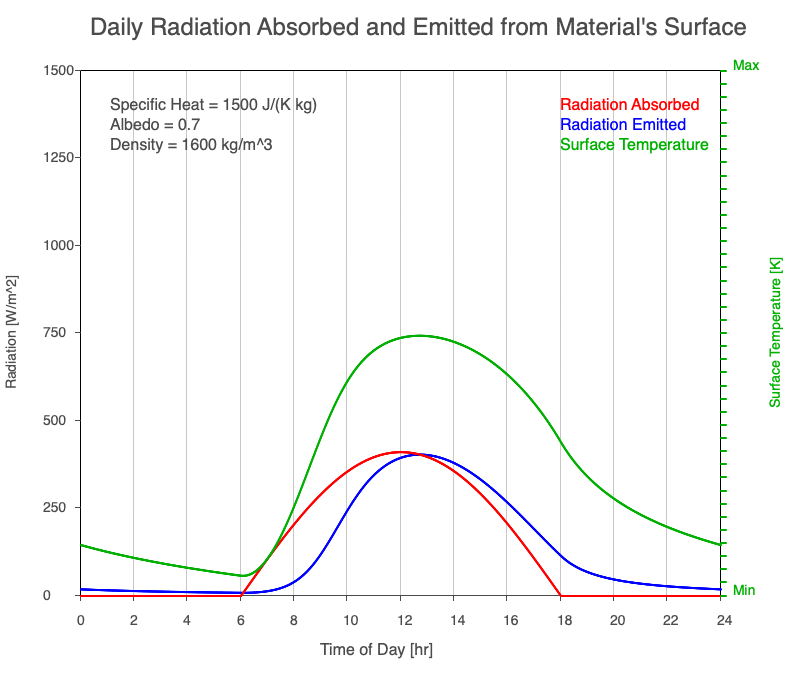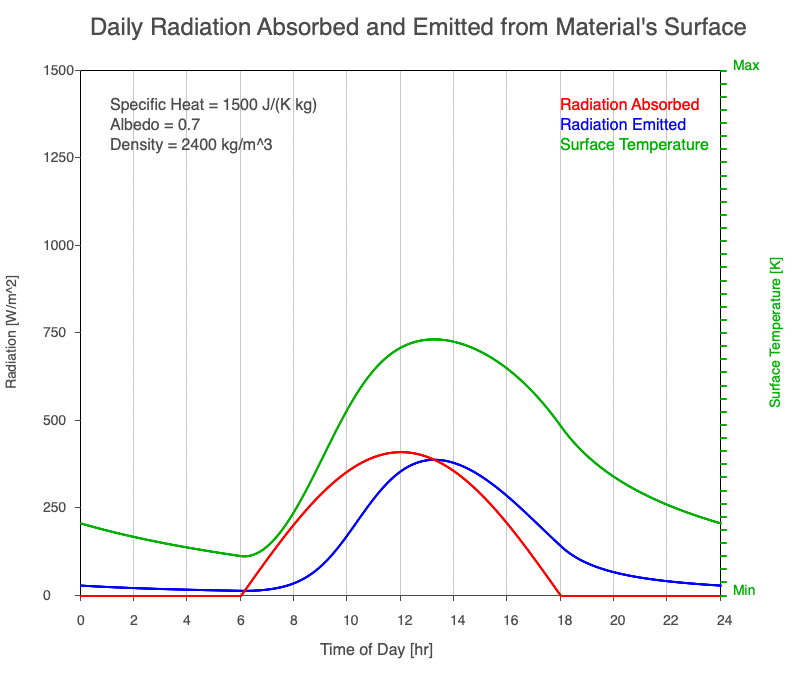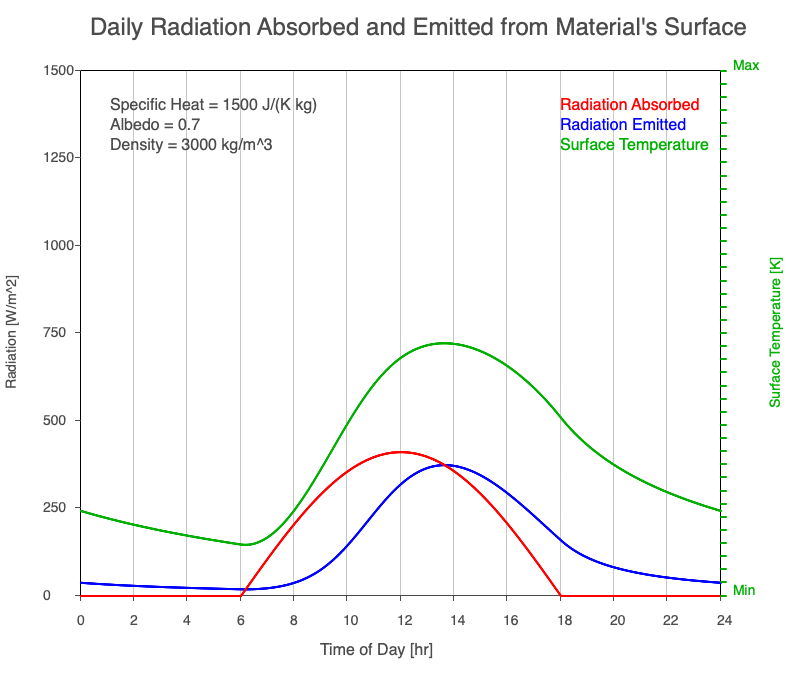
Diurnal Heating of Earth’s Surface Materials
It turns out water has extraordinary properties, and one is how much heat it takes to increase its temperature. Water in its liquid form has the highest specific heat for any common material found on Earth, so it takes much more heat to increase its temperature, as well as giving off a great deal of heat before its temperature drops one degree.
Grass has a high specific heat since it is made up of mostly liquid water. Notice that snow has a considerably lower specific heat than liquid water, so the phase of water is important to consider when analyzing a surface’s radiation budget.
Specific Heat of the Surface Materials
| Surface | Specific Heat [J/(K kg)] |
| Dry Soil | 800 |
| Grass | 3000 |
| Sand | 830 |
| Water | 4182 |
| Snow | 2090 |
| Pavement | 880 |
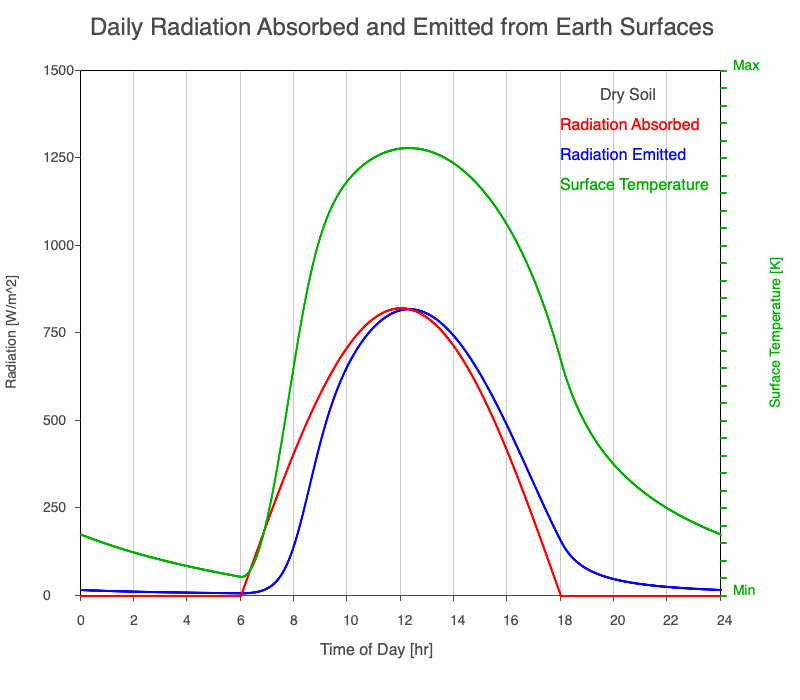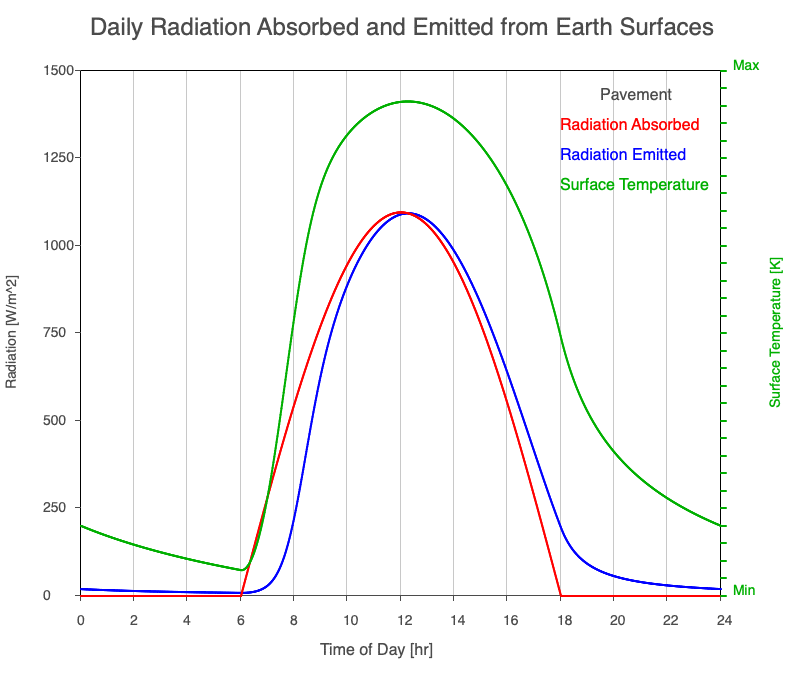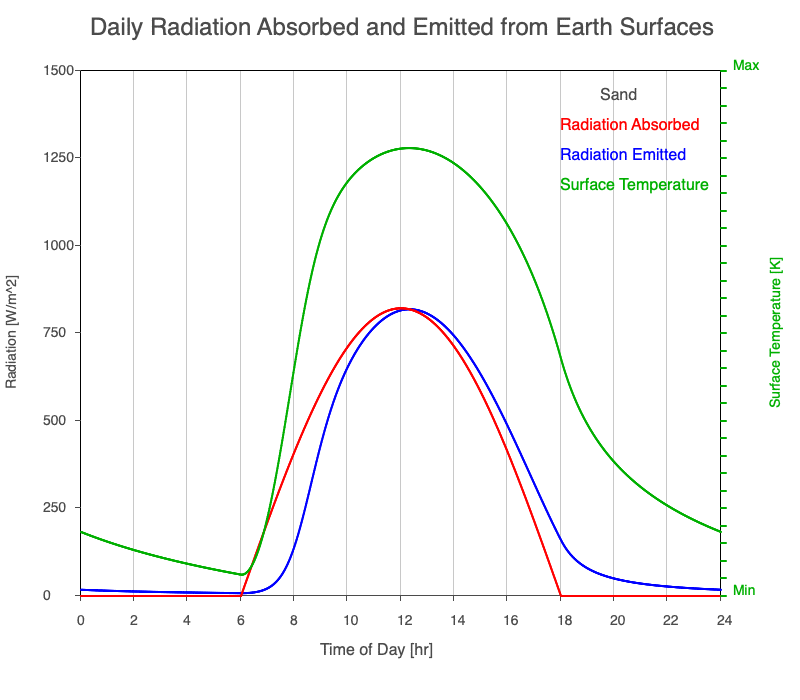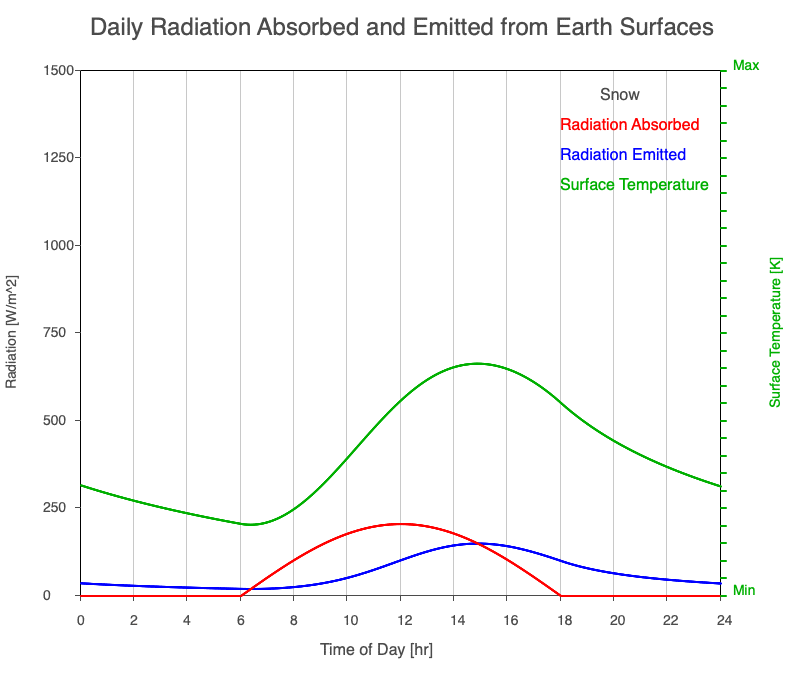
Animation showing the diurnal heating patterns for different types of surfaces on Earth. The red line is the radiation absorbed by the surface, the blue is the radiation emitted from the warmed surface, and the green line is the surface temperature.
Our Sun emits roughly 10% of its thermal radiation in the ultraviolet, 45% in visible light, and 45% in the near-infrared. Luckily our stratosphere absorbs most of the UV (see Atmospheric Layers for more detail), so primarily visible light and near-infrared reach the ground. There are six surfaces modeled in the animation: dry soil, grass, sand, water, snow, and pavement.
Reflectivity in Visible Light and Near-Infrared
| Surface | Visible Light | Near-Infrared |
| Dry Soil | Moderately Absorbent | Absorbent |
| Grass | Absorbent¹ | Reflective |
| Sand | Moderately Absorbent | Absorbent |
| Water² | Absorbent | Absorbent |
| Snow | Reflective | Reflective |
| Pavement | Absorbent | Absorbent |
¹Although plants are highly absorbent of visible light; the radiation is not converted to heat within the plant; rather, photosynthesis converts it to chemical energy.
² Because water is semi-transparent to sunlight, more than the surface is exposed to radiation. As a simple estimate, the radiation model assumes that all sunlight is homogeneously absorbed within 75 cm from the surface.
Activity: Find the Hottest and Coldest Places Outside during the Next 20 Minutes

Before the pandemic, inexpensive non-contact thermal infrared sensors were about $15 in hardware stores, but the prices have doubled to tripled recently. If you have one, pair up with a friend, make a map of your surroundings (preferably outside), and find the coldest and warmest places during the next 20 minutes. Even better is to challenge other teams to see who finds the hottest, coldest, and the largest range in temperature. Those who win usually apply their understanding of the factors that affect diurnal heating!
Big Ideas
- Materials may have quite different diurnal heating patterns when exposed to the same intensity of sunlight.
- More reflective objects absorb less energy from sunlight compared to more absorbent materials, so they stay cooler during the daytime.
- Specific heat is a measure of the amount of heat required to raise the temperature of a 1 kg mass by 1ºC.
- A material with a high specific heat needs more heat to raise the temperature compared to a substance with low specific heat, so it heats less during the daytime and cools less at night.
- A low-density material heats more quickly than a higher density substance during the day.
- How absorbed sunlight heats the Earth’s surface depends on a combination of its properties: reflectivity, specific heat, and density.

0 Comments