Radiation
Radiation is the emission and transmission of energy as electromagnetic waves or light. The unit particle of radiation is the photon, which is a massless packet of energy that behaves both as a particle and a wave. When an object emits a photon, it gives up energy, and when an object absorbs a photon, it gains energy. The photon’s energy may be converted to heat, but not always. During photosynthesis, for example, visible light is transformed into chemical energy.
A photon is often characterized based on its wavelength, frequency, and amplitude. The electromagnetic spectrum (EM spectrum) is the range of wavelengths and frequencies of all photons. Visible light is the only radiation that we can see with our eyes; we can’t see most of the vast EM spectrum. However, we use the entire range in many technologies: medical x-rays, ultraviolet sterilization of sewage, photography and movies, radio and microwave communication, etc.
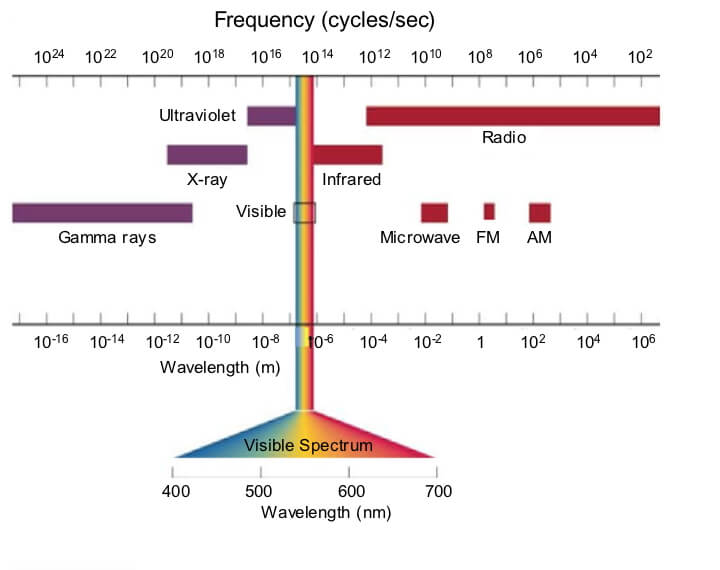
Diagram of the electromagnetic spectrum categorized by wavelength and frequency (the number of peaks or troughs passing a location per second).
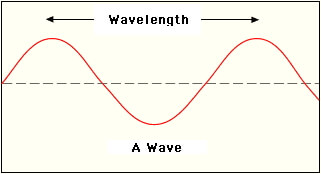
The distance between trough to trough or peak to peak on a wave is the wavelength.
Emission
There are five ways in which matter emits or gives off electromagnetic radiation.
- Electron shell change causes emission predominantly of ultraviolet and visible light.
- Molecular vibration generates mostly near-infrared.
- Molecular spin produces mostly far-infrared and radio waves.
- Nuclear reactions produce gamma rays and x-rays.
- Thermal radiation: the broad spectrum emitted from objects with a temperature above absolute zero (0 K). Blackbody radiation is the theoretical maximum thermal radiation emitted based on an object’s temperature. Blackbody radiation is a useful starting place to explore, but in nature, we experience thermal radiation since objects are not “perfect” emitters.
Emission reduces the energy of an object giving off photons. Once emitted, the radiation interacts with surrounding matter in three ways: absorption, reflection, and transmission.
This short movie from NASA is one of the best overviews I have seen, and the website has useful information on the electromagnetic spectrum.
Energy and Absorption, Reflection, and Transmission
- When matter captures or absorbs photons, the amount of energy within the material increases.
- When a photon bounces or reflects off the matter, the amount of energy within the material doesn’t change. Instead, the direction in which the photon is traveling changes.
- When a photon passes or transmits through the material’s matter, the amount of energy within the matter doesn’t change.
Supporting Article: Earth’s Energy Budget.
Thermal Radiation
Thermal radiation is an essential mechanism of heat transfer in Earth systems since it spans a large portion of the electromagnetic (EM) spectrum. There are several key relationships between the EM spectrum emitted from objects based on their temperature.
1) The wavelength of the radiation most emitted, referred to as the peak wavelength, decreases as the object warms (see the graph on the left below). This relationship is known as Wien’s Law.
2) The total amount of thermal radiation emitted increases rapidly as the object warms (see graph on the right below). This trend is known as the Stefan-Boltzmann Law.
3) As an object warms, it emits more thermal radiation at every wavelength (see the animation on the left below). So if you measured the intensity of a given wavelength emitted from one body and compared it to that of another, if the value of the first was higher, the first object is hotter than the second.
4) As a material warms, it emits a higher percentage of thermal radiation in shorter wavelengths (see the animation on the right below) and a lower proportion at longer wavelengths.
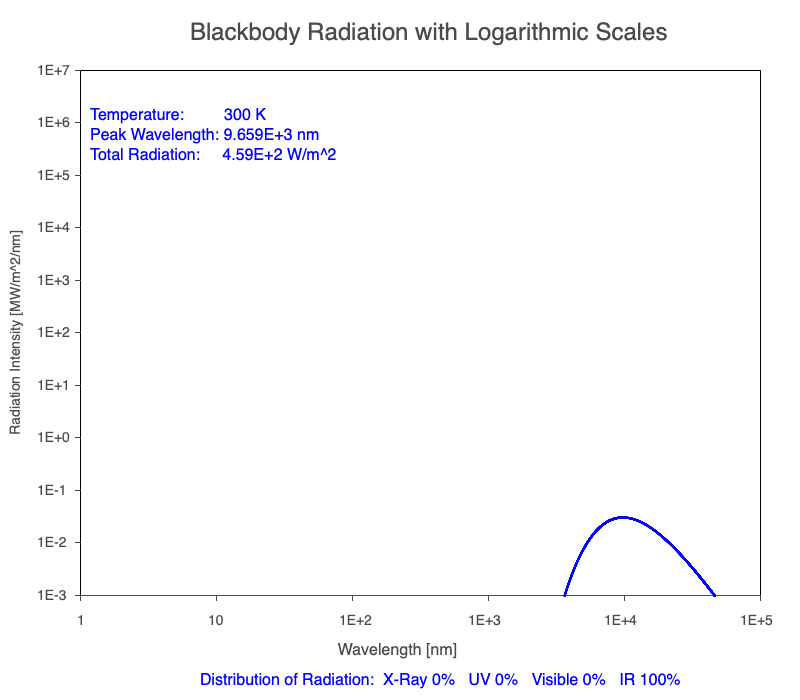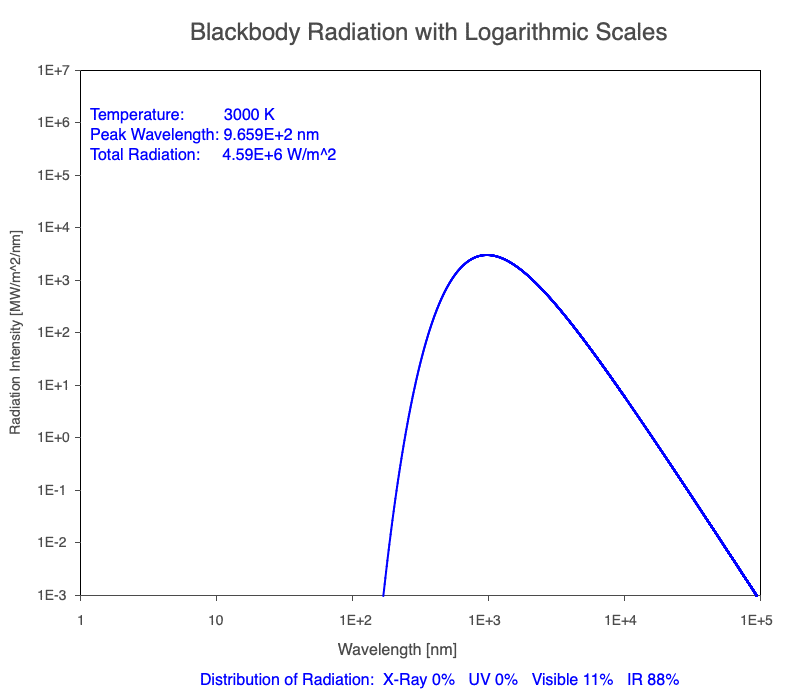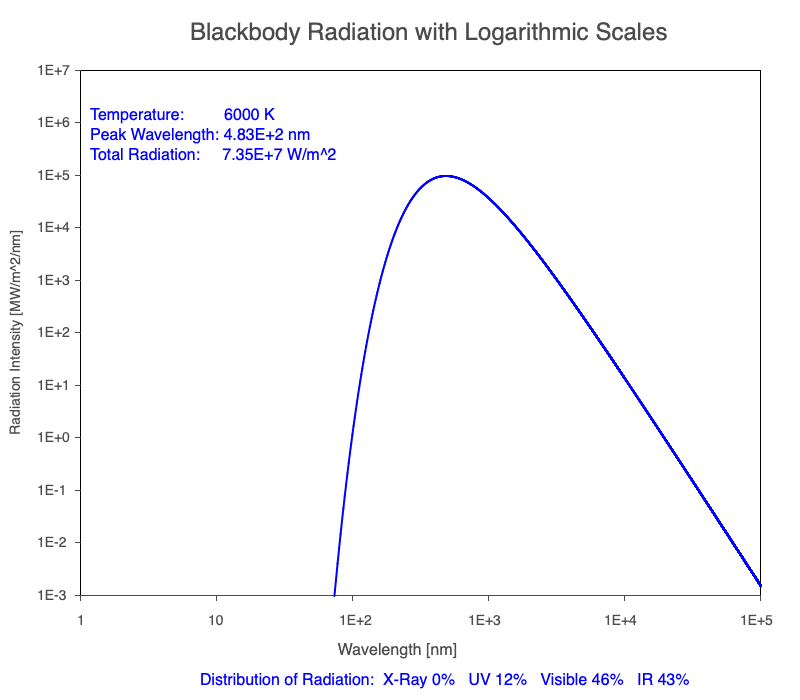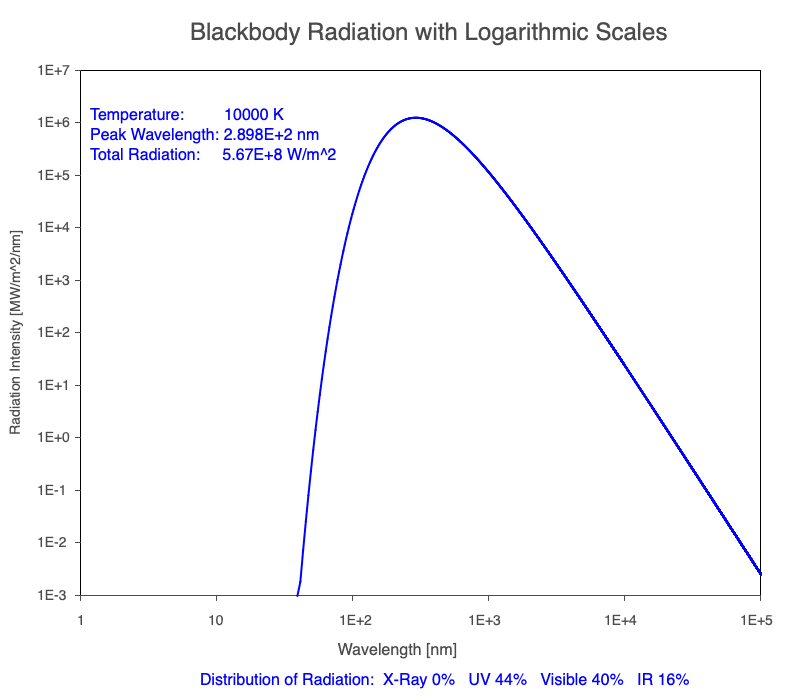
Animation of the thermal radiation spectrum emitted from objects of increasing temperature.
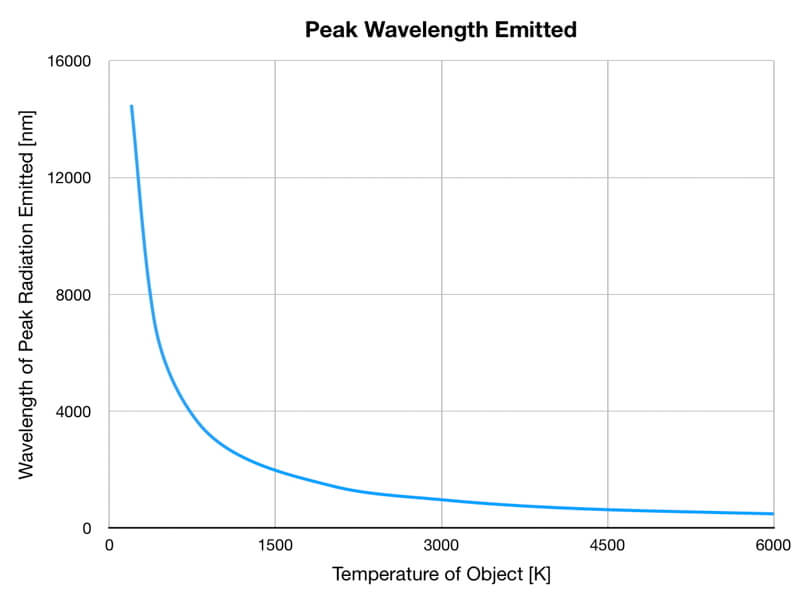
The peak wavelength emitted is inversely proportional to the object’s temperature.
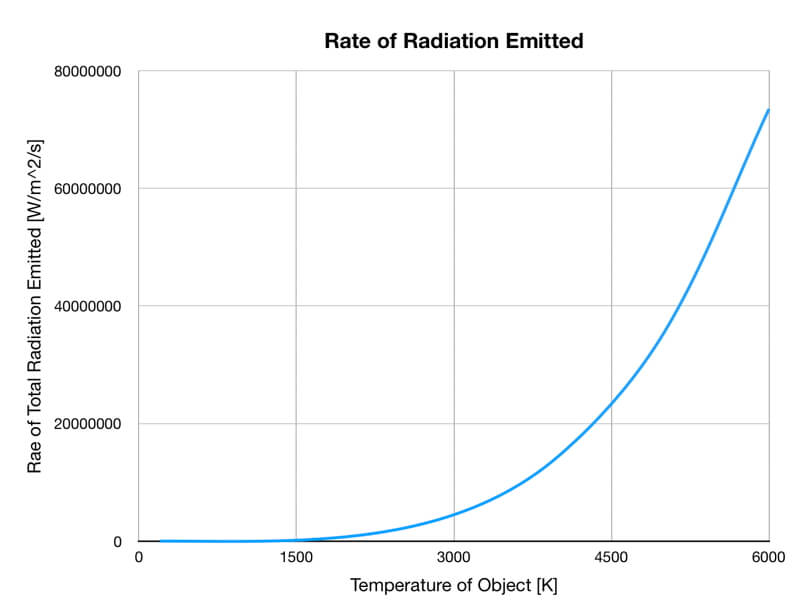
The rate of thermal radiation emitted from an object is proportional to the object’s temperature in degrees Kelvin to the fourth power.
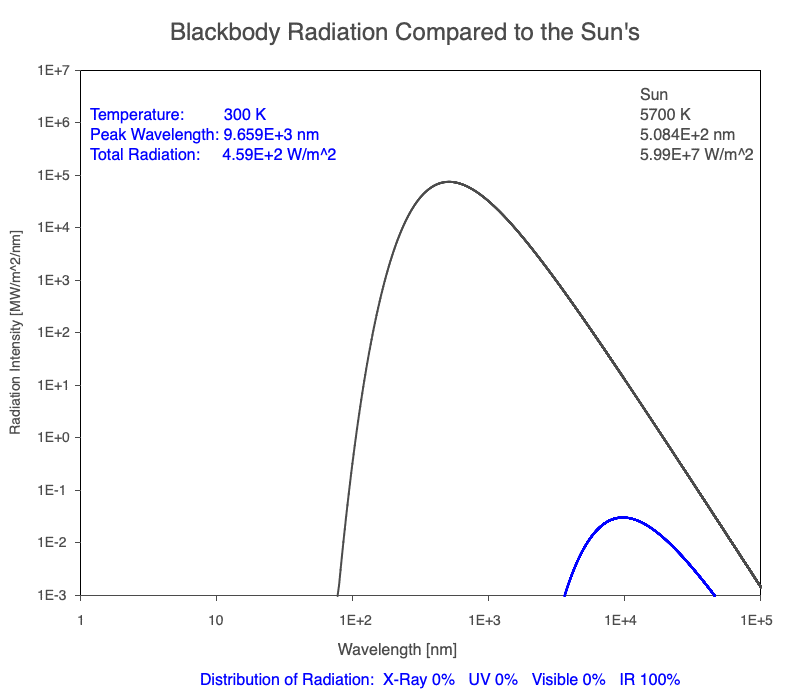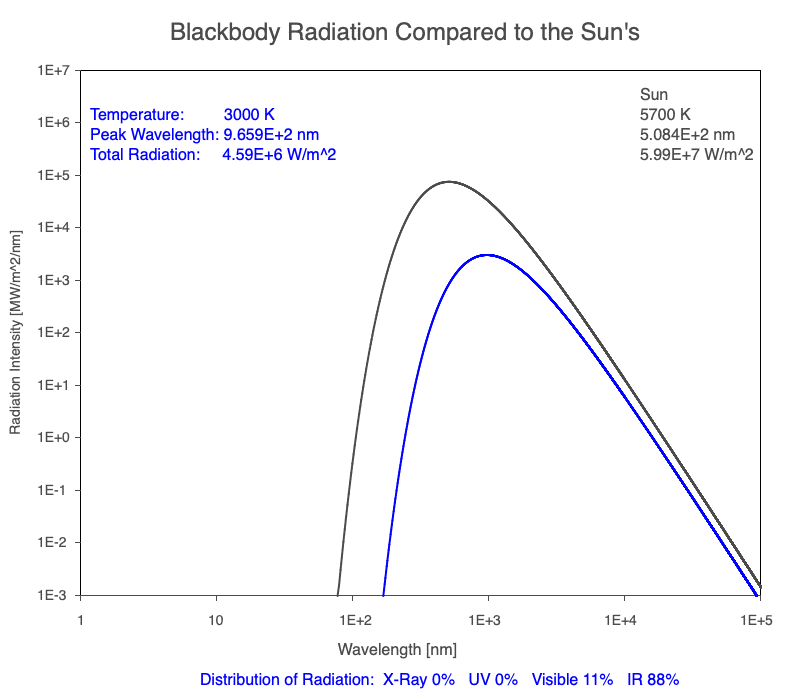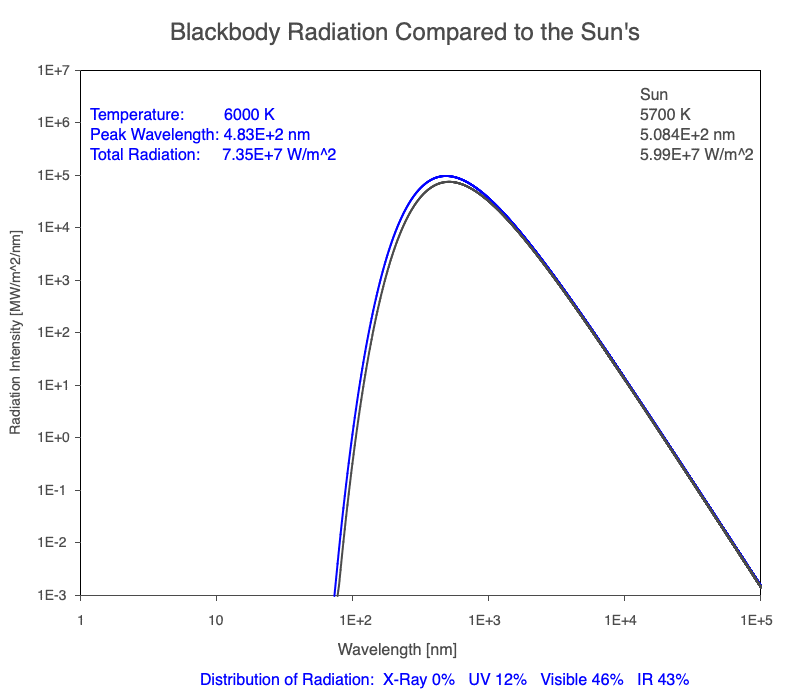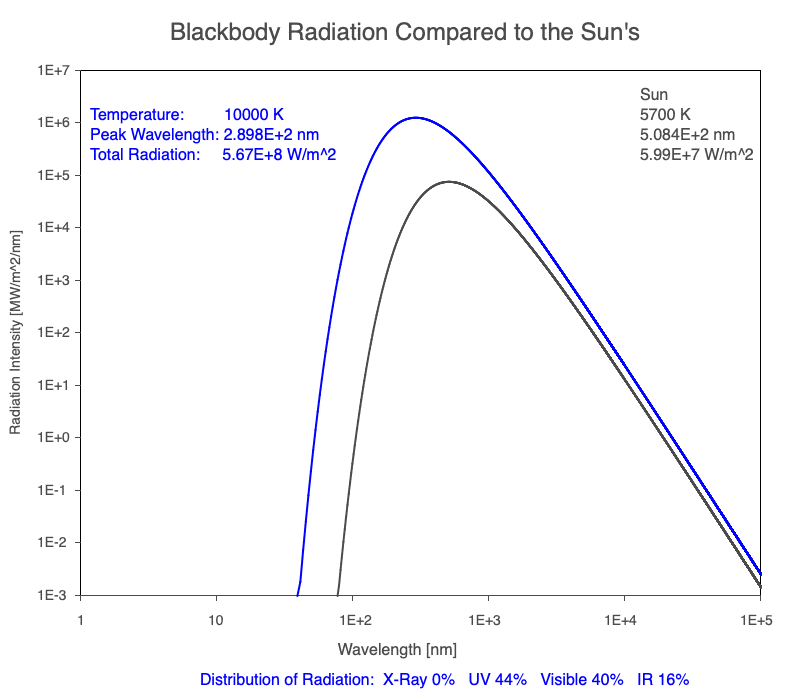
Compare the EM spectrum emitted (blue line) to that of the Sun (black line) as an object’s temperature increases. When cooler than the Sun, the object emits less radiation at all wavelengths. When warmer, the object emits more radiation at each wavelength.
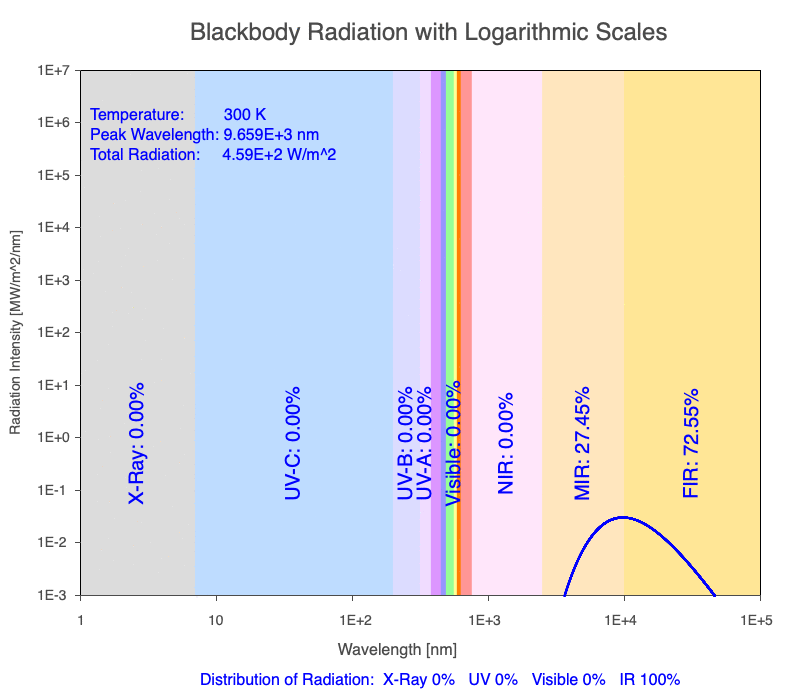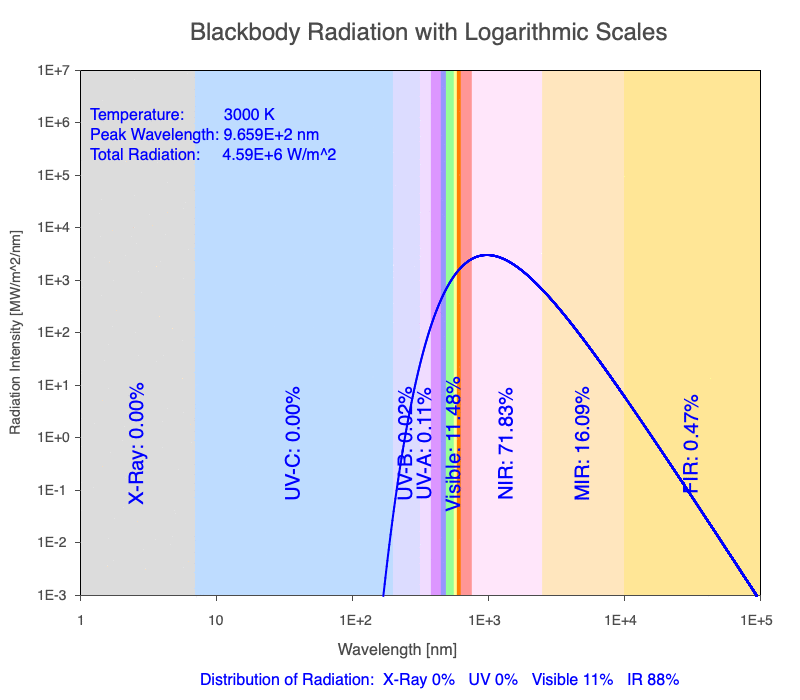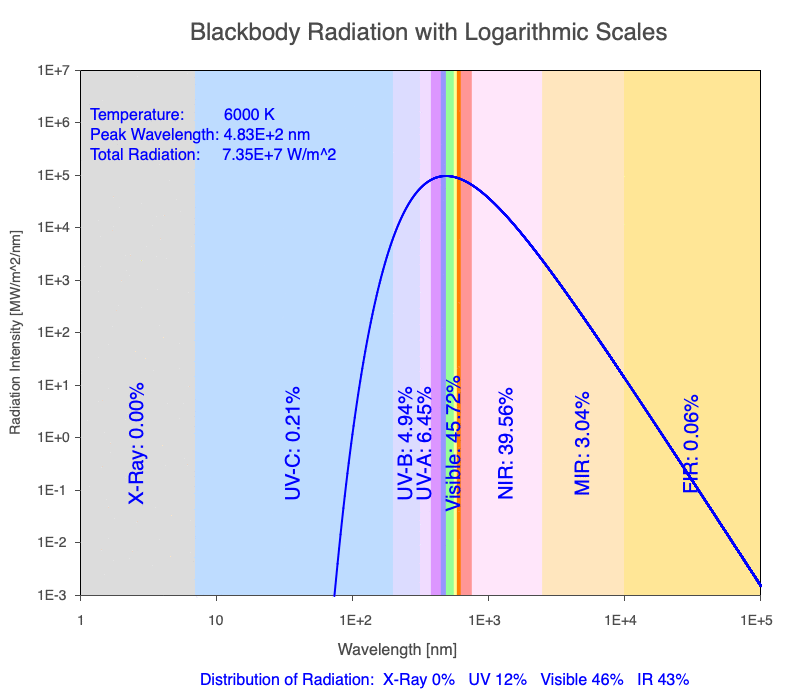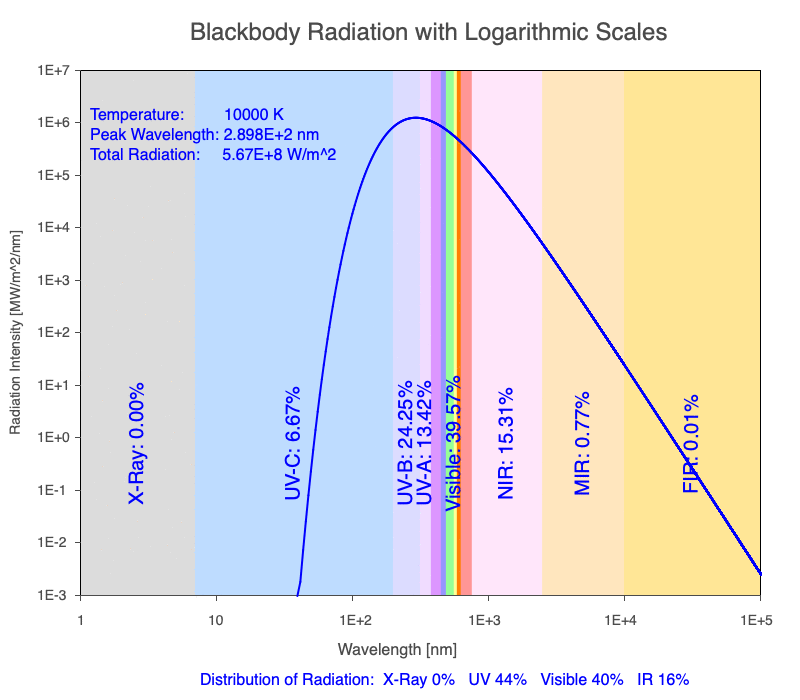
As an object warms, a greater percentage of the emitted spectrum is in shorter wavelengths.
Blackbody Radiation Desktop Software
Use the Blackbody Radiation software to explore the spectrum of radiation emitted from objects with a range of temperatures. The graphs used in the animations above were created using the software. To help, here is a document of key terms.
Note: A blackbody is a theoretical material that absorbs all radiation falling on it, reflecting or transmitting none. It is a hypothetical object that is a perfect absorber, which also makes it a perfect emitter of radiation over all wavelengths.
Big Ideas
- Radiation is the emission and transmission of energy as electromagnetic waves or light.
- A photon, which is a massless packet of energy that behaves both as a particle and a wave, is the unit particle of radiation.
- The electromagnetic spectrum (EM spectrum) is the range of wavelengths and frequencies of all photons.
- There are five ways in which matter emits or gives off electromagnetic energy.
- Thermal radiation is the broad spectrum emitted from objects with a temperature above absolute zero (0 K).
- The wavelength of the radiation most emitted, referred to as the peak wavelength, decreases as the object warms.
- The total amount of thermal radiation emitted increases rapidly as the object warms.
- As an object warms, it emits more thermal radiation at every wavelength.
- Emission takes energy away from the object that is emitting photons, reducing the total energy of the object.
- When matter captures or absorbs photons, the amount of energy within the material increases.
- When a photon bounces or reflects off the matter, the amount of energy within the material doesn’t change.
- When a photon passes or transmits through the material’s matter, the amount of energy within the matter doesn’t change.

0 Comments